When 16.3 g of an organic compound known to be 57.15% C, 4.8% H, and 38.06% O by mass is dissolved in 700.7 g of water, the freezing point is −0.343 ∘C. The normal freezing point of water is 0 ∘C. What is the molecular formula for the organic compound? Assume that the organic compound is a molecular solid and does not ionize in water. ?f values for various solvents are given in the colligative constants table
When 16.3 g of an organic compound known to be 57.15% C, 4.8% H, and 38.06% O by mass is dissolved in 700.7 g of water, the freezing point is −0.343 ∘C. The normal freezing point of water is 0 ∘C. What is the molecular formula for the organic compound? Assume that the organic compound is a molecular solid and does not ionize in water. ?f values for various solvents are given in the colligative constants table
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 128IP: Some nonelectrolyte solute (molar mass = 142 g/mol) was dissolved in 150. mL of a solvent (density =...
Related questions
Question
When 16.3 g of an organic compound known to be 57.15% C, 4.8% H, and 38.06% O by mass is dissolved in 700.7 g of water, the freezing point is −0.343 ∘C. The normal freezing point of water is 0 ∘C. What is the molecular formula for the organic compound? Assume that the organic compound is a molecular solid and does not ionize in water. ?f values for various solvents are given in the colligative constants table.
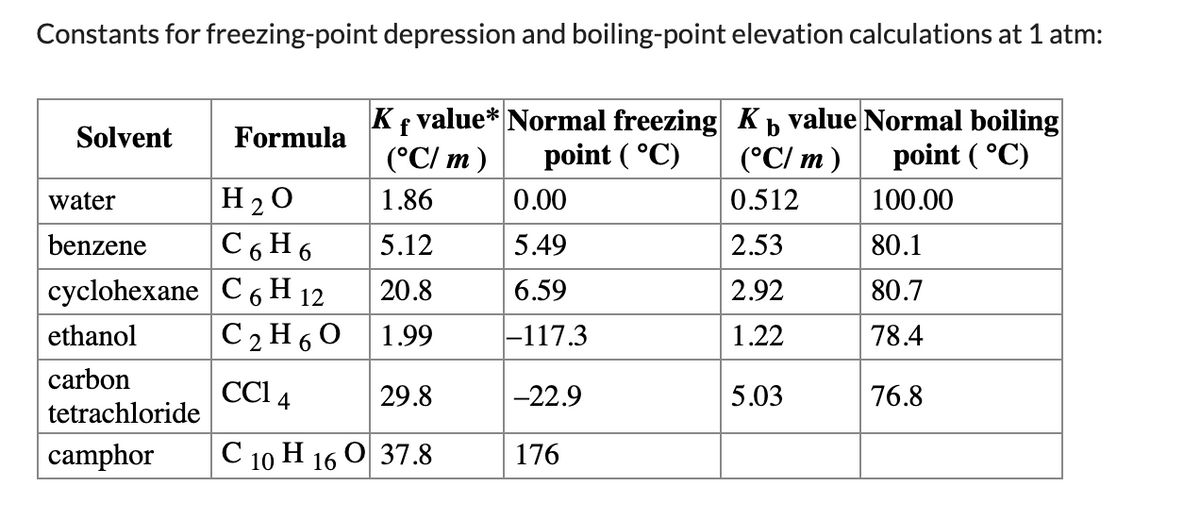
Transcribed Image Text:Constants for freezing-point depression and boiling-point elevation calculations at 1 atm:
K
f value* Normal freezing K value Normal boiling
Solvent
Formula
(°C/ m )
point ( °C)
(°C/ m)
point ( °C)
water
H20
1.86
0.00
0.512
100.00
C 6 H 6
cyclohexane C 6 H 12
C2 H 6 0
benzene
5.12
5.49
2.53
80.1
20.8
6.59
2.92
80.7
ethanol
1.99
|-117.3
1.22
78.4
carbon
СС 4
29.8
-22.9
5.03
76.8
tetrachloride
camphor
С 10 Н 16 0 37.8
176
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,