14. How do partial charges affect attractions between water molecules? Draw sev molecules, and use dotted lines to draw where one molecule is attracted to anoth molecule. (You can either insert a Google Doc Drawing or you can draw it on eithe your whiteboard, take a picture, and then insert into the blue box below.) Insert Drawing Here 15. How does this help us explain why water molecules stick together? BACK TO THE SIMULATION (Check out mineral oil, again in "opaque" surface vi- 16. Is mineral oil a symmetrical molecule? Does it have partial charges?
14. How do partial charges affect attractions between water molecules? Draw sev molecules, and use dotted lines to draw where one molecule is attracted to anoth molecule. (You can either insert a Google Doc Drawing or you can draw it on eithe your whiteboard, take a picture, and then insert into the blue box below.) Insert Drawing Here 15. How does this help us explain why water molecules stick together? BACK TO THE SIMULATION (Check out mineral oil, again in "opaque" surface vi- 16. Is mineral oil a symmetrical molecule? Does it have partial charges?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 18P: A gaseous binary compound has a vapor density that is 2.53 times that of nitrogen at 100°C and...
Related questions
Question
100%
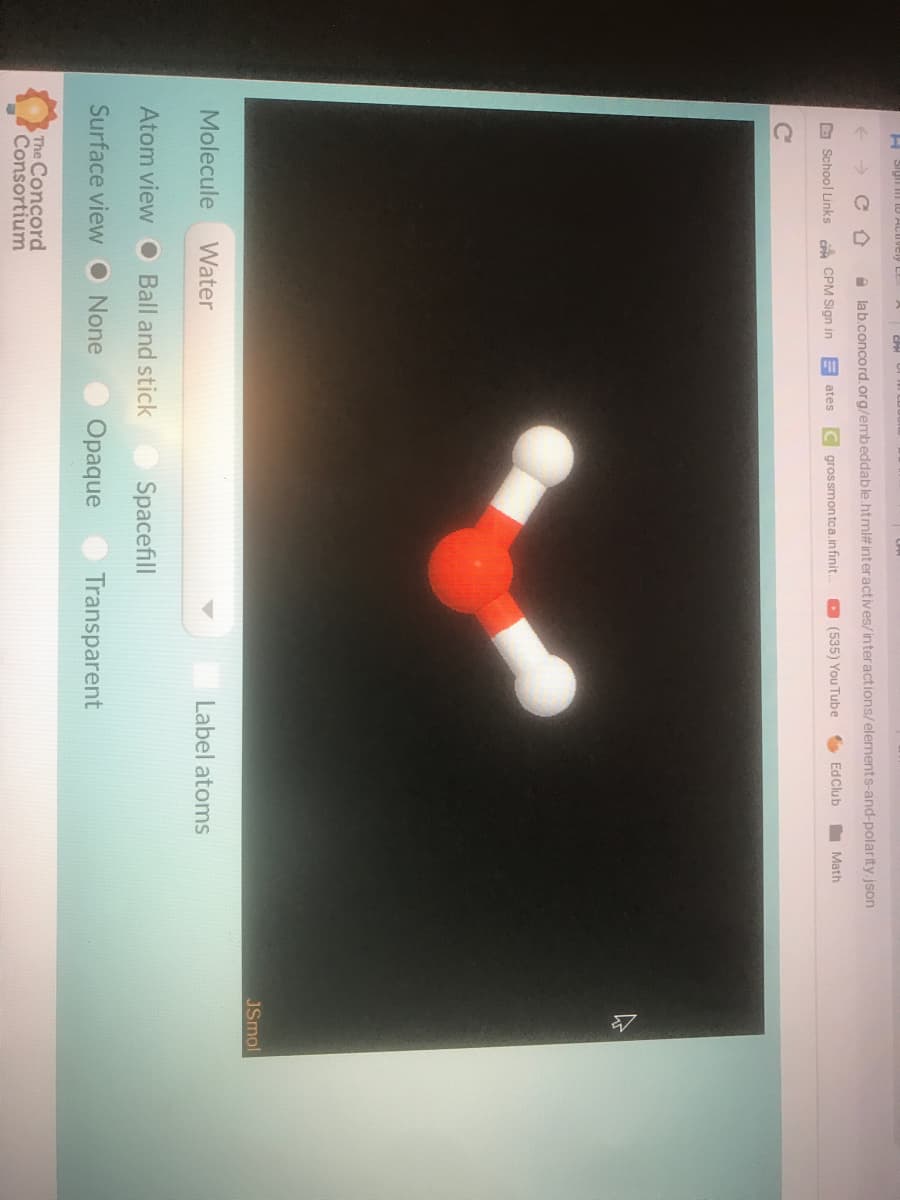
Please question 14, 15 and 16 if you cant do all of them dont do anything just leave it. The another image for question 16

Transcribed Image Text:w oppositely charged particles?
13. If we put these ideas together, how can we explain the partial negative charge on oxygen
and the partial positive charge on hydrogen?
14. How do partial charges affect attractions between water molecules? Draw several vwater
molecules, and use dotted lines to draw where one molecule is attracted to another
molecule. (You can either insert a Google Doc Drawing or you can draw it on either paper or
your whiteboard, take a picture, and then insert into the blue box below.)
Insert Drawing Here
15. How does this help us explain why water molecules stick together?
BACK TO THE SIMULATION (Check out mineral oil, again in "opaque" surface view.)
16. Is mineral oil a symmetrical molecule? Does it have partial charges?
17. Think back to lesson 3 and when we put drops of liquids on pennies. Could we get many
drops of oil to stick on a penny? What can we infer about attractions between mineral oil
Transcribed Image Text:H Sigin in to
A lab.concord.org/embeddable.html# interactives/interactions/elements-and-polarity .json
O School Links c CPM Sign in
E ates
C grossmon tca.in finit.
O (535) You Tube
6 EdClub
Math
JSmol
Molecule
Water
Label atoms
Atom view
Ball and stick
Spacefill
Surface view O None
Opaque
Transparent
The Concord
Consortium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
