Table 1. Vapor Pressure of Water at Different lem Temperature, °C PH3Os mm Hg Temperature, C PHo, mm Hg 16°C 13.6 22 °C 19.8 17°C 14.5 23 °C 21.1 18°C 15.5 24 °C 22.4 19 °C 16.5 25 °C 23.8 20 °C 17.5 26 °C 25.2 21 °C 18.7 27 °C 26.7 A reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 mL of hydrogen gas was collected by water displacement in a 22 °C water bath. The barometric pressure gas. The in the lab that day was 746 mm Hg. 1. Use Dalton's law and the vapor pressure of water at 22 °C (Table 1) to calculate the partial pressure of hydrogen gas in the gas collecting tube. 2. Use the combined gas law to calculate the "corrected" volume of hydrogen at STP. Hint: Watch your units for temperature and pressure! 3. What is the theoretical number of moles of hydrogen that can be produced from 0.028 g of Mg? Hint: Refer to Equation I for the balanced equation for the reaction. 4. Divide the corrected volume of hydrogen by the theoretical number of moles of hydrogen to calculate the molar volume (in L/mol) of hydrogen at STP.
Table 1. Vapor Pressure of Water at Different lem Temperature, °C PH3Os mm Hg Temperature, C PHo, mm Hg 16°C 13.6 22 °C 19.8 17°C 14.5 23 °C 21.1 18°C 15.5 24 °C 22.4 19 °C 16.5 25 °C 23.8 20 °C 17.5 26 °C 25.2 21 °C 18.7 27 °C 26.7 A reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 mL of hydrogen gas was collected by water displacement in a 22 °C water bath. The barometric pressure gas. The in the lab that day was 746 mm Hg. 1. Use Dalton's law and the vapor pressure of water at 22 °C (Table 1) to calculate the partial pressure of hydrogen gas in the gas collecting tube. 2. Use the combined gas law to calculate the "corrected" volume of hydrogen at STP. Hint: Watch your units for temperature and pressure! 3. What is the theoretical number of moles of hydrogen that can be produced from 0.028 g of Mg? Hint: Refer to Equation I for the balanced equation for the reaction. 4. Divide the corrected volume of hydrogen by the theoretical number of moles of hydrogen to calculate the molar volume (in L/mol) of hydrogen at STP.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 38P
Related questions
Question
Please answer 1, 2 and 3 as we are allowed to ask 3 questions at once please and thank you!!
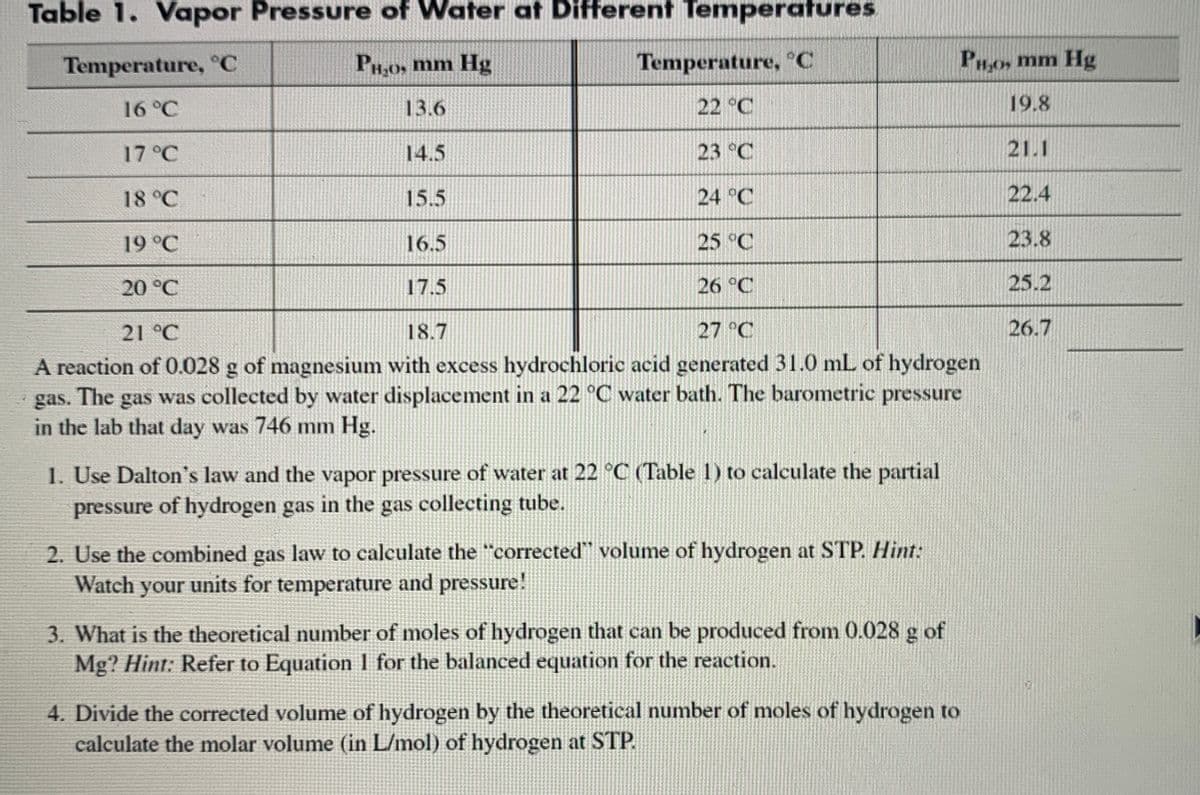
Transcribed Image Text:Table 1. Vapor Pressure of Water at Different Temperatures
Temperature, C
PH,0, mm Hg
Temperature, °C
PHo, mm Hg
16°C
13.6
22 °C
19.8
17 °C
14.5
23 °C
21.1
18 °C
15.5
24 °C
22.4
19 °C
16.5
25 °C
23.8
20 °C
17.5
26 °C
25.2
21 °C
18.7
27 °C
26.7
A reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 mL of hydrogen
gas. The gas was collected by water displacement in a 22 °C water bath. The barometric pressure
in the lab that day was 746 mm Hg.
1. Use Dalton's law and the vapor pressure of water at 22 °C (Table 1) to calculate the partial
pressure of hydrogen gas in the gas collecting tube.
2. Use the combined gas law to calculate the "corrected" volume of hydrogen at STP. Hint:
Watch your units for temperature and pressure!
3. What is the theoretical number of moles of hydrogen that can be produced from 0.028 g of
Mg? Hint: Refer to Equation 1 for the balanced equation for the reaction.
4. Divide the corrected volume of hydrogen by the theoretical number of moles of hydrogen to
calculate the molar volume (in L/mol) of hydrogen at STP.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning