14.53 How much energy is released in the fission of 1 kg of 235 U according to the equation below? The experimen- tally determined masses of the products are 136.92532 u and 96.91095 u, respectively. (The masses of 235 U and the neutron are given in Example Problem 14.6.) 235U + n →¹3Te + Zr + 2 n 92 52
14.53 How much energy is released in the fission of 1 kg of 235 U according to the equation below? The experimen- tally determined masses of the products are 136.92532 u and 96.91095 u, respectively. (The masses of 235 U and the neutron are given in Example Problem 14.6.) 235U + n →¹3Te + Zr + 2 n 92 52
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter19: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 72AE
Related questions
Question
14.53 Round your answers to 6 decimal places. Box your final answers.
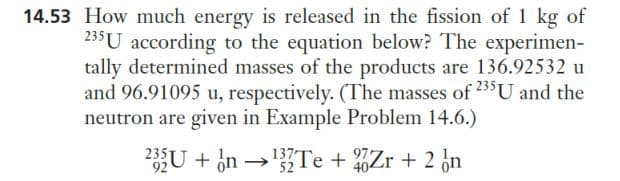
Transcribed Image Text:14.53 How much energy is released in the fission of 1 kg of
235 U according to the equation below? The experimen-
tally determined masses of the products are 136.92532 u
and 96.91095 u, respectively. (The masses of 235 U and the
neutron are given in Example Problem 14.6.)
235U + n →¹3Te + Zr + 2 n
92
52
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning