Chemistry please neat writing! 13.28 Pick an appropriate solvent(s) from table to dissolve each of the following substances. Common Polar Solvents Common Nonpolar Solvents water hexane (C6H12) (H₂O) acetone diethyl ether (CH3CH₂OCH2CH3) (CH3COCH3) methyl alcohol (CH3OH) toluene (C7H8) Part A glucose (polar) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. ΑΣΦ ? Part B salt (ionic) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. O | ΑΣΦΑ ΑΣΦΑΤΑ Ο ? Part C vegetable oil (nonpolar) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. ΑΣΦ 4 ? ▼ Part D sodium nitrate (ionic) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. °= | ΑΣΦ → C ? Y
Chemistry please neat writing! 13.28 Pick an appropriate solvent(s) from table to dissolve each of the following substances. Common Polar Solvents Common Nonpolar Solvents water hexane (C6H12) (H₂O) acetone diethyl ether (CH3CH₂OCH2CH3) (CH3COCH3) methyl alcohol (CH3OH) toluene (C7H8) Part A glucose (polar) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. ΑΣΦ ? Part B salt (ionic) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. O | ΑΣΦΑ ΑΣΦΑΤΑ Ο ? Part C vegetable oil (nonpolar) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. ΑΣΦ 4 ? ▼ Part D sodium nitrate (ionic) Express your answer as a chemical formula. If there is more than one answer, separate them by a comma. °= | ΑΣΦ → C ? Y
Chapter11: Organic Compounds: Alkanes
Section: Chapter Questions
Problem 11.66E
Related questions
Question
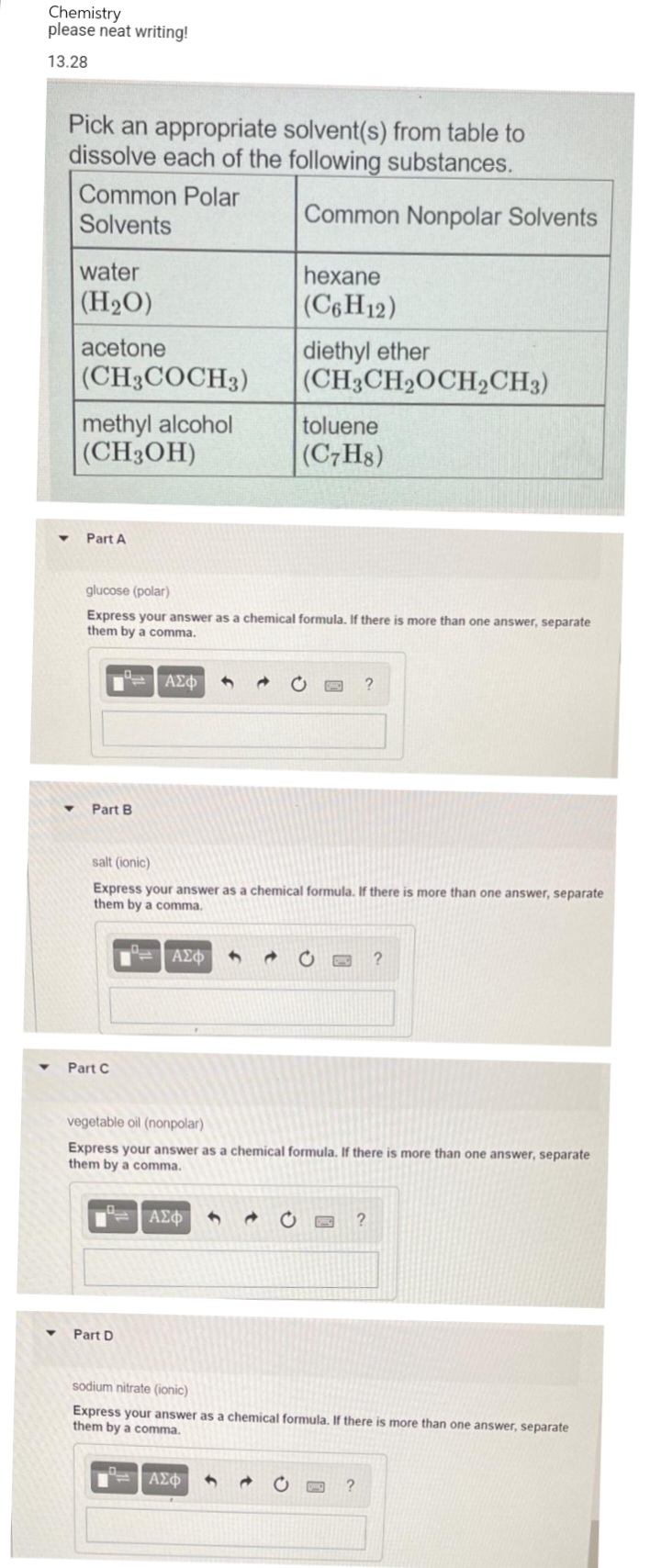
Transcribed Image Text:Chemistry
please neat writing!
13.28
Pick an appropriate solvent(s) from table to
dissolve each of the following substances.
Common Polar
Solvents
Common Nonpolar Solvents
water
hexane
(H₂O)
(C6H12)
acetone
diethyl ether
(CH3CH2OCH2CH3)
(CH3COCH3)
methyl alcohol
(CH3OH)
toluene
(C7H8)
Part A
glucose (polar)
Express your answer as a chemical formula. If there is more than one answer, separate
them by a comma.
ΑΣΦ
?
▾ Part B
salt (ionic)
Express your answer as a chemical formula. If there is more than one answer, separate
them by a comma.
0= | ΑΣΦΑ
ΑΣΦΑ
?
Part C
vegetable oil (nonpolar)
Express your answer as a chemical formula. If there is more than one answer, separate
them by a comma.
ΑΣΦ ↑
?
▼ Part D
sodium nitrate (ionic)
Express your answer as a chemical formula. If there is more than one answer, separate
them by a comma.
°= | ΑΣΦ
?
Y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,