15.13 Suppose that the gas-phase reactions A B and A are both elementary processes with rate con- stants of 4.7 x 103s1 and 5.8 x 101 s, respectively. (a) What is the value of the equilibrium constant for the equilibrium A (g) =B(g)? (b) Which is greater at equi- librium, the partial pressure of A or the partial pressure of B? B
15.13 Suppose that the gas-phase reactions A B and A are both elementary processes with rate con- stants of 4.7 x 103s1 and 5.8 x 101 s, respectively. (a) What is the value of the equilibrium constant for the equilibrium A (g) =B(g)? (b) Which is greater at equi- librium, the partial pressure of A or the partial pressure of B? B
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.29P: 7-29 The following reaction was allowed to reach equilibrium at 25°C. Under each component is its...
Related questions
Question
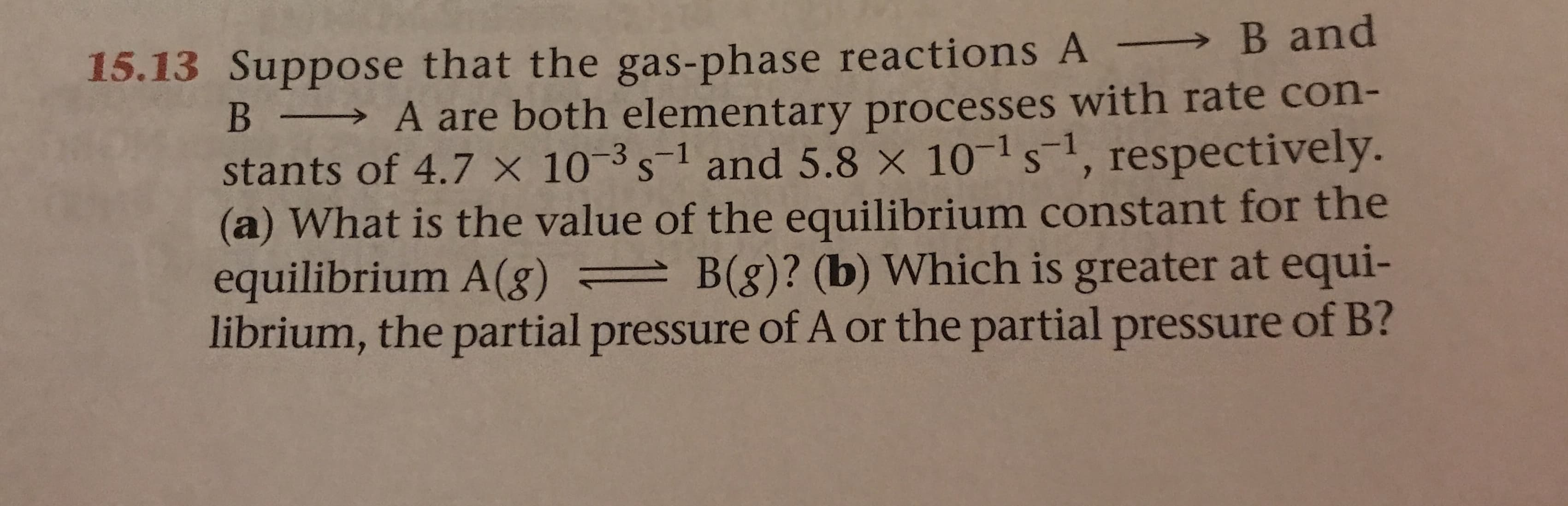
Transcribed Image Text:15.13 Suppose that the gas-phase reactions A B and
A are both elementary processes with rate con-
stants of 4.7 x 103s1 and 5.8 x 101 s, respectively.
(a) What is the value of the equilibrium constant for the
equilibrium A (g) =B(g)? (b) Which is greater at equi-
librium, the partial pressure of A or the partial pressure of B?
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 5 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,