Q: Determine the point groups for H -C = C - CH3.
A: We see that propyne is linear. To find the point group for linear molecules, we use the following…
Q: 1. What are symmetry elements? What is their significance?
A: Group theory is a branch of study that deal with the algebraic structures. The concept of group…
Q: Determine the point group of the molecule shown. The answer should have the correct formatting for…
A:
Q: Determine the symmetry elements of BrF3 E, 20v C2, 20v O E. C2 E, C2, 20v
A:
Q: 1. Identify all symmetry elements in the following molecules. Use diagrams or verbal descriptions…
A: In chemistry, a symmetry element is a point, line or place about which symmetry operation can be…
Q: Prepare a representation flowchart for trans- N2F2, which has C2h symmetry.
A: N2F2 is the chemical formula of Dinitrogen difluoride. It exists as gas state at room temperature…
Q: 6. Which symmetry elements are lost on going from (a) BC13 to BCl2F and (b) BC12F to BBRCIF. Which…
A:
Q: What groups and the symmetry elements
A: 1. C2V Point group : Symmetry elements : C2v group as it has the symmetry elements E, C2, and two…
Q: molecule or object and show all symmetry elements explicitly. • a wine glass • cis-platin…
A: The principle axis denotes the highest order of the axis (360/ n) through which operation, an…
Q: (i) Determine the point group for each compound below. O,N O,N -NO2 -NO2 NO2 O2N (ii) Using symmetry…
A: Solution: 1. Nitrobenzene have C2v point group as principle axis passing from C4 carbon and N atom.…
Q: Using symmetry determine how many vibrational modes the following species have and how many and…
A: The explanation is given below-
Q: List all the symmetry elements of the following molecules and determine their point groups. a)…
A: The molecule contains the following symmetry elements: 1,2,4-trichlorobenzene has a plane of…
Q: Determine all the symmetry elements of the following molecules Allene (C3H4)
A: Structure of the allene: The central carbon of allene is sp hybridized and two terminal carbon are…
Q: 2) Consider the molecule BF3: a) Draw the molecule-showing-axes b) What is the highest symmetry…
A:
Q: Q1: What will degree of freedom (trans, rot & vib) for the following molecules: a) NO, b) so, c)…
A: question 1. "Since there are multiple sub-parts in this question and you have mentioned to solve all…
Q: (a) Identify the principal (z) rotational axis for all molecules provided below. (b) Provide a…
A: Symmetry operation is an action perfomed on a molecule , yields an new orientation of it that is…
Q: Assign a point group to each of the molecules below. Determine is the molecule is chiral be drawing…
A: A symmetry point group is a systematic nomenclature of molecules on the basis of symmetry. In order…
Q: Benzene ring has only one s6 symmetry but 6c2 in addition to other symmetry elements True False
A: The ‘s’notation symbolizes improper axis of symmetry which includes an axis of symmetry followed by…
Q: Write all symmetry elements and point group of PCI5 molecule. (Draw the shape
A: Write all symmetry elements and point group of PCI5 molecule ?
Q: Which of the following would be expected to have a dipole moment of zero on the basis of symmetry?…
A: The molecule that is expected to have a dipole moment of zero on the basis of symmetry has to be…
Q: Draw an MO diagram for the pi-bonding in 1,3-butadiene (C2h symmetry). Draw a picture of each MO…
A: M.O CAN BE OBTAINED FROM NOMOF PARTICIPATING ORBITALS,HERE TOTAL 4 ATOM PARTICIPATED SO 4 M.O WILL…
Q: Sketch the following molecules or ions, include the important symmetry elements on the drawing and…
A: The molecular structure of a molecule/ion provides information about the arrangement of the…
Q: 5. Using diagrams with numbered atoms, illustrate the symmetry operations of the three-fold proper…
A:
Q: What is name of the rotation axis of highest n in the molecule 1,3-cyclobutadiene ?
A: To find the axis of highest n in the molecule 1,3-cyclobutadiene
Q: What is the symmetry number of CH3Cl
A: The number of diverse but indistinguishable or similar configurations of an item that is the order…
Q: How many degrees of vibrational freedom does Al2Cl6 possess? Use the D2h character table to…
A: Vibrational degrees of freedom of Al2Cl6 are calculated as
Q: The types of symmetry operations for AsClz are: Select one: A. E, C3, C2 oy B. E, C3, C2 S2 C. E,…
A: We have to predict the symmetry elements in given compound.
Q: How many vibrational modes does an SO3 molecule have a) in the plane of the nuclei, b) perpendicular…
A: Sulphur trioxide (SO3) is a trigonal planar molecule. It has D3h symmetry with C 3 principle axis.…
Q: What can you conclude about the symmetry of the C3H5+ ion as it actually exists, as regards the bond…
A: We will discuss about the symmetry of the C3H5+ ion as it actually exists, as regards the bond…
Q: 10. Find the total number of symmetry elements in each of the fourteen Bravais lattices,
A: Lattice is an array of points showing how molecules or atoms are arranged in different sites.
Q: Which of the following would be expected to have a dipole moment of zero on the basis of symmetry?…
A: All symmetrical molecules have diople moment equal to zero and are considered as non-polar whereas…
Q: Write reasonable mechanisms for each of the following transformations and label any…
A:
Q: (d). From the side: Down the axis: What is the symmetry designation for this orbital: o, ,0. , Tg ,…
A:
Q: Under C4v symmetry, which irreducible representations, if any, correspond to an IR active vibration…
A: For a molecule to be vibrational active, check the irreducible representations corresponding to x, y…
Q: How many symmetry planes does p-dibromobenzene have? O 1 2 3 4
A: A plane of symmetry is a symmetry element. The plane of symmetry is defined as a flat surface that…
Q: st coplanar u
A:
Q: Benzene ring has only one S6 symmetry but bC2 in addition to other symmetry elements True False
A: Benzene belongs to D6h point group.
Q: The symmetry operation S is the same as the symmetry operation Select one OTrue False
A: The symmetry operation in S62 = C62 × perpendicular plane
Q: Determine the symmetry of the IR active modes for cyclohexane for boat conformation
A:
Q: CoA (CP)4
A: Let us 1st draw its structure and then its symmetry elements and then we can find its point group.
Q: Using appropriate diagram(s), show which of the symmetry operation(s) does the [SO4]2- ion have: (i)…
A:
Q: The complete set of symmetry elements in CHC13 are: Select one: O E, C3, 30y O C3, 30v, S3 O E, C3,…
A:
Q: Do these molecules has centre of symmetry(C.O.S)? If not,why? If we draw lines passing through the…
A: If an imaginary plane is passed to the any point of the surface through the center and a similar…
Q: Determine the symmetry of the IR active C-H stretching modes for cyclohexane boat conformation
A:
Q: Cl CI CI H CI
A: Symmetry of a molecule provides information about its resemblance to other part of the molecule. The…
Q: Would the coordination step between Br- and (CH3)3C+ be allowed if Br- were to approach from within…
A: Carbocation (CH3)3C+ has a triangular planar geometry and all three -CH3 groups are connected to…
Q: 3. Pick two of the three molecules below. Determine their point groups and whether they are chiral.…
A: At first one has to determine the symmetry elements present in the molecule molecule s. then from…
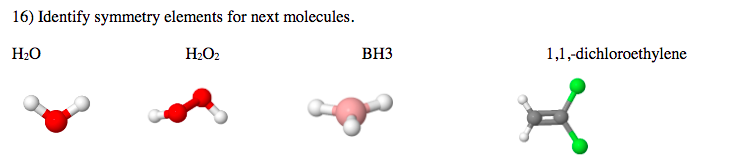
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

- a In the Td point group, an S41 improper rotation is equivalent to what other improper rotation? b In the D6h point group, the symmetry operation labeled C21 is equivalent to what other symmetry operation?Determine the D3h symmetry species of the sp3d hybrid orbitals, assuming that the C3 axis is coincident with the z-axis and that one of the orbitals lies along the positive x-axis. See Example 13.16.Identify the symmetry elements present in the following objects. a A ream of blank paper, no holes. b A ream of blank three-holed paper. c A round pencil, unsharpened, with cylindrical eraser. d A round pencil, sharpened, with cylindrical eraser.
- In your own words, explain why an object that has more symmetry elements is said to have higher symmetry than an object with fewer symmetry elements.Determine the point groups of the following molecules. a Hydrogen selenide, H2Se b Partially deuterated hydrogen sulfide, or HDS c The chair conformer of cyclohexane, C6H12 d The boat conformer of cyclohexane, C6H12a Unlike methane, bromochlorofluoromethane (CHBrClF) is chiral. Determine all symmetry elements that are present in CHBrClF and identify its point group. b If the fluorine in this molecule were substituted with a hydrogen atom, what is the point group for the new molecule? Is it chiral?
- Determine the symmetry species of the D3h point group for the sp2 hybrid orbitals, assuming that the C3 axis is coincident with the z-axis and that one of the orbitals lies along the positive x-axis. See Example 13.16.Determine which single symmetry operation of the following point groups is equivalent to the given combination of multiple symmetry operations. a In C2v, C2v=? b In C2h, iC2=? c In D6h, C6h=? d In D2d, C2C2=? e In Oh, iS4=?What symmetry elements must be present for a particular moleculeto be achiral?
- Identify the symmetry species of the Symmetry Adapted Linear Combination (SALC) ? = ?′ − ?′′ in the C2v molecule NO2, where ?′ is a 2px orbital on one O atom and ?′′ is a ???? 2px orbital on the other O atom.How many symmetry planes and symmetry axis in C3H8O. also is there a center of symmetry in C3H8O?Identify the symmetry species of the Symmetry Adapted Linear Combination (SALC) ? = ?? ′ − ?? ′′ in the C2v molecule NO2, where ?? ′ is a 2px orbital on one O atom and ?? ′′ is a 2px orbital on the other O atom. please show your reasonings from the basics thank you



