16. An aliquot of a 8.50 M stock aqueous solution of sodium chloride is used to create 800. mL of a 0.100 M dilute solution. What is the mass (in g) of sodium chloride present in the dilute solution? A: 4,68
16. An aliquot of a 8.50 M stock aqueous solution of sodium chloride is used to create 800. mL of a 0.100 M dilute solution. What is the mass (in g) of sodium chloride present in the dilute solution? A: 4,68
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 126AP: You pour 150.0 mL of a 0.250 M lead(ll) nitrate solution into an empty 500-mL flask. What is the...
Related questions
Question

Transcribed Image Text:16. An aliquot of a 8.50 M stock aqueous solution of sodium chloride is used to create 800. mL of a
0.100 M dilute solution. What is the mass (in g) of sodium chloride present in the dilute solution?
A: 4,68
Expert Solution
Step 1
Molarity: It is defined as the moles of solute dissolved in per litre of the solution or it is the amount of substance present in a certain volume of solution.
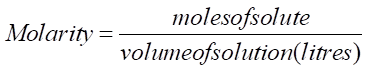
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning