Mass of KI tablet 0.425 g Mass of thoroughly dried filter paper 1.462 g Mass of filter paper + precipitate after first drying 1.775 g Mass of filter paper + precipitate after second drying 1.699 g Mass of filter paper + precipitate after third drying 1.698 g A student is given the task of determining the I content of tablets that contain Kl and an inert, water-soluble sugar as a filler. A tablet is dissolved in 50.0 mL of distilled water, and an excess of 0.20 MPb(NO3)2(aq) is added to the solution. A yellow precipitate forms, which is then filtered, washed, and dried. The data from the experiment are shown in the table above. a. For the chemical reaction that occurs when the precipitate forms, i. write a balanced, net-ionic equation for the reaction, and ii. explain why the reaction is best represented by a net-ionic equation. b. Explain the purpose of drying and weighing the filter paper with the precipitate three times. c. In the filtrate solution, is [K"] greater than, less than, or equal to [NO3 ]? Justify your answer. d. Calculate the number of moles of precipitate that is produced in the experiment. e. Calculate the mass percent of I in the tablet. f. In another trial, the student dissolves a tablet in 55.0 mL of water instead of 50.0 mL of water. Predict whether the experimentally determined mass percent of I will be greater than, less than, or equal to the amount calculated in part (e). Justify your answer. g. A student in another lab also wants to determine the | content of a Kl tablet but does not have access to Pb(NO3)2 - However, the student does have access to 0.20 MAGNO3 , which reacts with I (aq) to produce Agl(s). The value of Ksp for Agl is 8.5 x 107. i. Will the substitution of AgNO3 for Pb(NO3)2 result in the precipitation of thel ion from solution? Justify your answer. ii. The student only has access to one KI tablet and a balance that can measure to the nearest 0.01 g. Will the student be able to determine the mass of Agl produced to three significant figures? Justify your answer.
Mass of KI tablet 0.425 g Mass of thoroughly dried filter paper 1.462 g Mass of filter paper + precipitate after first drying 1.775 g Mass of filter paper + precipitate after second drying 1.699 g Mass of filter paper + precipitate after third drying 1.698 g A student is given the task of determining the I content of tablets that contain Kl and an inert, water-soluble sugar as a filler. A tablet is dissolved in 50.0 mL of distilled water, and an excess of 0.20 MPb(NO3)2(aq) is added to the solution. A yellow precipitate forms, which is then filtered, washed, and dried. The data from the experiment are shown in the table above. a. For the chemical reaction that occurs when the precipitate forms, i. write a balanced, net-ionic equation for the reaction, and ii. explain why the reaction is best represented by a net-ionic equation. b. Explain the purpose of drying and weighing the filter paper with the precipitate three times. c. In the filtrate solution, is [K"] greater than, less than, or equal to [NO3 ]? Justify your answer. d. Calculate the number of moles of precipitate that is produced in the experiment. e. Calculate the mass percent of I in the tablet. f. In another trial, the student dissolves a tablet in 55.0 mL of water instead of 50.0 mL of water. Predict whether the experimentally determined mass percent of I will be greater than, less than, or equal to the amount calculated in part (e). Justify your answer. g. A student in another lab also wants to determine the | content of a Kl tablet but does not have access to Pb(NO3)2 - However, the student does have access to 0.20 MAGNO3 , which reacts with I (aq) to produce Agl(s). The value of Ksp for Agl is 8.5 x 107. i. Will the substitution of AgNO3 for Pb(NO3)2 result in the precipitation of thel ion from solution? Justify your answer. ii. The student only has access to one KI tablet and a balance that can measure to the nearest 0.01 g. Will the student be able to determine the mass of Agl produced to three significant figures? Justify your answer.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 94AP
Related questions
Question
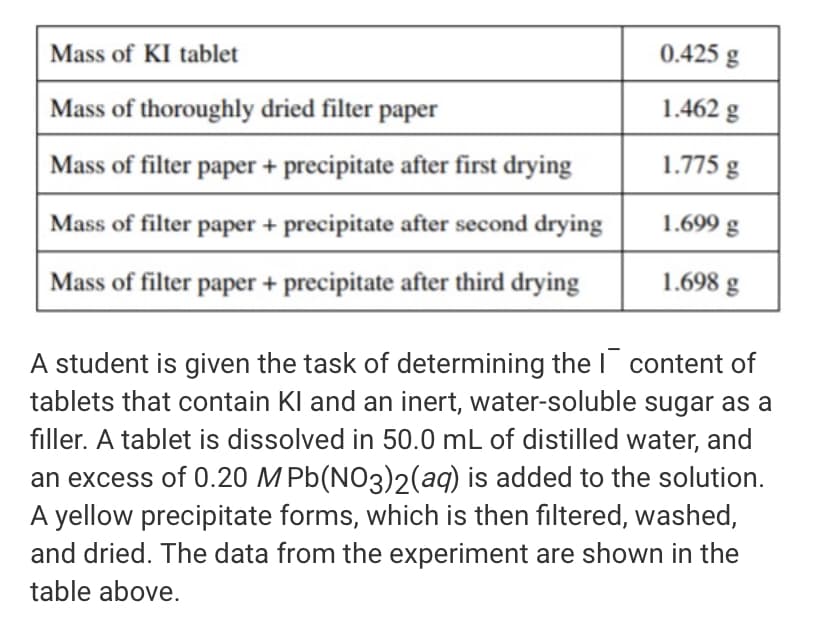
Transcribed Image Text:Mass of KI tablet
0.425 g
Mass of thoroughly dried filter paper
1.462 g
Mass of filter paper + precipitate after first drying
1.775 g
Mass of filter paper + precipitate after second drying
1.699 g
Mass of filter paper + precipitate after third drying
1.698 g
A student is given the task of determining the I content of
tablets that contain Kl and an inert, water-soluble sugar as a
filler. A tablet is dissolved in 50.0 mL of distilled water, and
an excess of 0.20 MPb(NO3)2(aq) is added to the solution.
A yellow precipitate forms, which is then filtered, washed,
and dried. The data from the experiment are shown in the
table above.
![a. For the chemical reaction that occurs when the
precipitate forms,
i. write a balanced, net-ionic equation for the
reaction, and
ii. explain why the reaction is best represented by
a net-ionic equation.
b. Explain the purpose of drying and weighing the filter
paper with the precipitate three times.
c. In the filtrate solution, is [K"] greater than, less than, or
equal to [NO3 ]? Justify your answer.
d. Calculate the number of moles of precipitate that is
produced in the experiment.
e. Calculate the mass percent of I in the tablet.
f. In another trial, the student dissolves a tablet in 55.0
mL of water instead of 50.0 mL of water. Predict
whether the experimentally determined mass percent
of I will be greater than, less than, or equal to the
amount calculated in part (e). Justify your answer.
g. A student in another lab also wants to determine the
| content of a Kl tablet but does not have access to
Pb(NO3)2 - However, the student does have access to
0.20 MAGNO3 , which reacts with I (aq) to produce
Agl(s). The value of Ksp for Agl is 8.5 x 107.
i. Will the substitution of AgNO3 for Pb(NO3)2
result in the precipitation of thel ion from
solution? Justify your answer.
ii. The student only has access to one KI tablet
and a balance that can measure to the nearest
0.01 g. Will the student be able to determine the
mass of Agl produced to three significant
figures? Justify your answer.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2de8c41c-c6ff-4208-826f-5d77798ed91c%2F92c8383b-f635-43f2-9a22-30a7735dba1f%2Fx1ipu1.jpeg&w=3840&q=75)
Transcribed Image Text:a. For the chemical reaction that occurs when the
precipitate forms,
i. write a balanced, net-ionic equation for the
reaction, and
ii. explain why the reaction is best represented by
a net-ionic equation.
b. Explain the purpose of drying and weighing the filter
paper with the precipitate three times.
c. In the filtrate solution, is [K"] greater than, less than, or
equal to [NO3 ]? Justify your answer.
d. Calculate the number of moles of precipitate that is
produced in the experiment.
e. Calculate the mass percent of I in the tablet.
f. In another trial, the student dissolves a tablet in 55.0
mL of water instead of 50.0 mL of water. Predict
whether the experimentally determined mass percent
of I will be greater than, less than, or equal to the
amount calculated in part (e). Justify your answer.
g. A student in another lab also wants to determine the
| content of a Kl tablet but does not have access to
Pb(NO3)2 - However, the student does have access to
0.20 MAGNO3 , which reacts with I (aq) to produce
Agl(s). The value of Ksp for Agl is 8.5 x 107.
i. Will the substitution of AgNO3 for Pb(NO3)2
result in the precipitation of thel ion from
solution? Justify your answer.
ii. The student only has access to one KI tablet
and a balance that can measure to the nearest
0.01 g. Will the student be able to determine the
mass of Agl produced to three significant
figures? Justify your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT