16. To what minimum temperature would 200. grams of water need to be heated in order for 120. grams of ammonium chloride to completely dissolve? (~68°C) 60 Ntly Cl 120 9 NH₂ Cl 55° C ond -
16. To what minimum temperature would 200. grams of water need to be heated in order for 120. grams of ammonium chloride to completely dissolve? (~68°C) 60 Ntly Cl 120 9 NH₂ Cl 55° C ond -
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 114AP
Related questions
Question
how do you do question number 16? i have attempted it but am not getting the right answer. this is a non graded practice worksheet
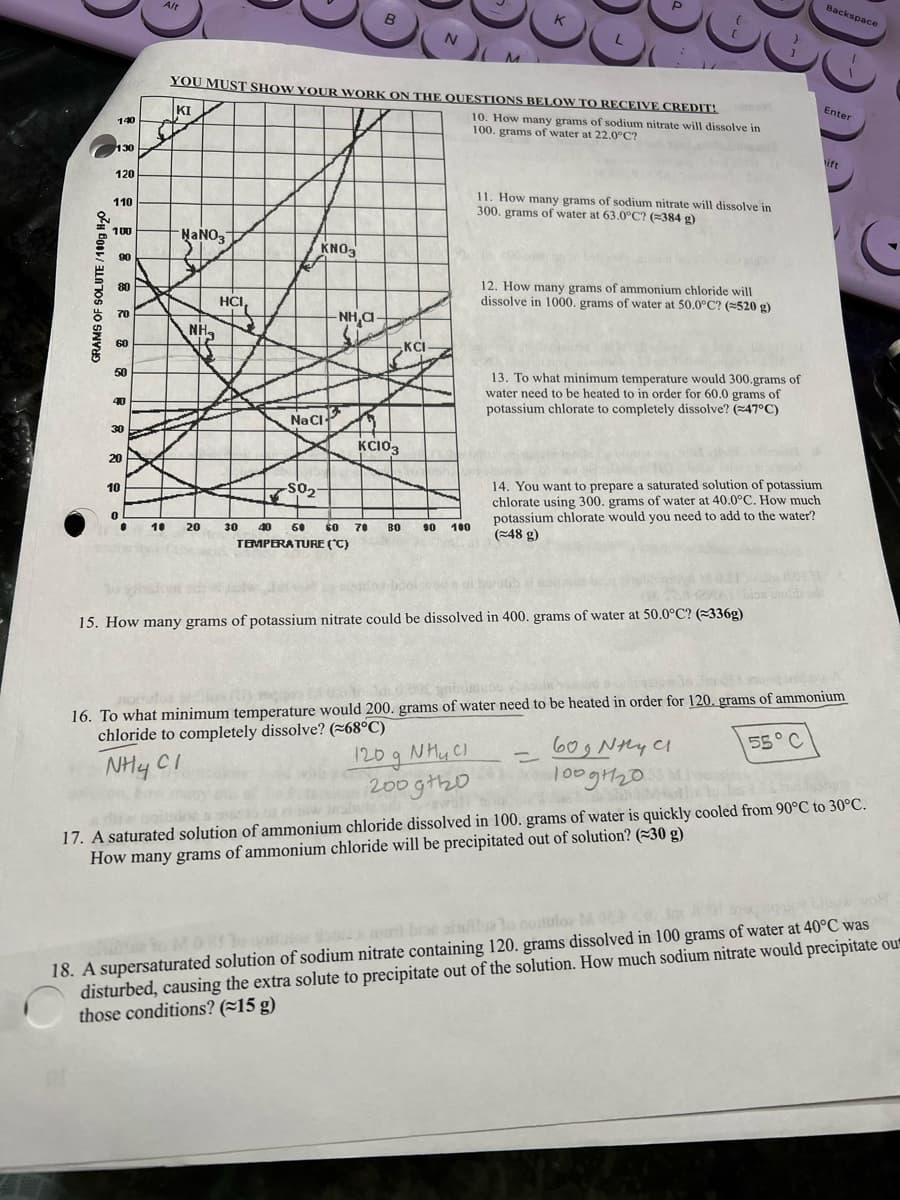
Transcribed Image Text:GRAMS OF SOLUTE/100g H₂0
140
130
120
110
100
90
80
70
60
50
40
30
20
10
0
●
10
Alt
NaNO3
NH₂
20
YOU MUST SHOW YOUR WORK ON THE QUESTIONS BELOW TO RECEIVE CREDIT!
KI
10. How many grams of sodium nitrate will dissolve in
100. grams of water at 22.0°C?
HCI,
e
KNO3
Na Cl
-SO₂
B
NH,C
KC103
30 40 50 60 70 ВО
TEMPERATURE (°C)
N
KCI
M
90 100
K
11. How many grams of sodium nitrate will dissolve in
300. grams of water at 63.0°C? (≈384 g)
12. How many grams of ammonium chloride will
dissolve in 1000. grams of water at 50.0°C? (=520 g)
13. To what minimum temperature would 300.grams of
water need to be heated to in order for 60.0 grams of
potassium chlorate to completely dissolve? (47°C)
14. You want to prepare a saturated solution of potassium
chlorate using 300. grams of water at 40.0°C. How much
potassium chlorate would you need to add to the water?
(48 g)
15. How many grams of potassium nitrate could be dissolved in 400. grams of water at 50.0°C? (336g)
Backspace
=
Enter
ift
16. To what minimum temperature would 200. grams of water need to be heated in order for 120. grams of ammonium
chloride to completely dissolve? (~68°C)
NH CI
120 g NH₂ Cl
200gth20
55° C
60g Nhy Cl
100g1120
die
17. A saturated solution of ammonium chloride dissolved in 100. grams of water is quickly cooled from 90°C to 30°C.
How many grams of ammonium chloride will be precipitated out of solution? (30 g)
doos mont bie shillos tonosuloz M 021
O
18. A supersaturated solution of sodium nitrate containing 120. grams dissolved in 100 grams of water at 40°C was
disturbed, causing the extra solute to precipitate out of the solution. How much sodium nitrate would precipitate out
those conditions? (~15 g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning