165 mL of a 0.905 M HCl solution Express your answer to three significant figures and include the appropriate units. V 01 HA Value 1 mL OWC Submit Previous Answers Request Answer ? X Incorrect; Try Again; 6 attempts remaining
165 mL of a 0.905 M HCl solution Express your answer to three significant figures and include the appropriate units. V 01 HA Value 1 mL OWC Submit Previous Answers Request Answer ? X Incorrect; Try Again; 6 attempts remaining
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter14: Acid-base Equilibria
Section: Chapter Questions
Problem 52E: Which of the following will increase the percent of HF that is converted to the fluoride ion in...
Related questions
Question
3
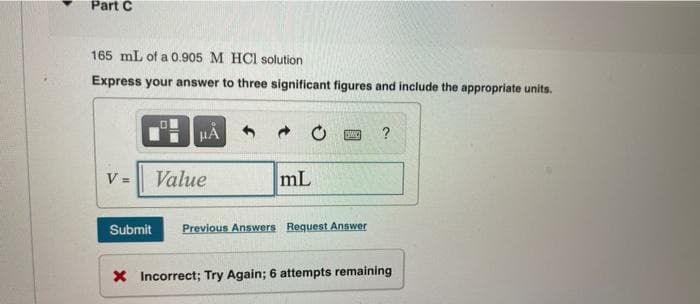
Transcribed Image Text:Part C
165 mL of a 0.905 M HCl solution
Express your answer to three significant figures and include the appropriate units.
V =
μÅ
Value
mL
SMIC
Submit Previous Answers Request Answer
?
X Incorrect; Try Again; 6 attempts remaining

Transcribed Image Text:MISSED THIS? Watch (WE 13.7; Read Section 13.8. You can
click on the Review link to access the section in your eText.
Determine the volume of 0.165 M NaOH solution required to
neutralize each sample of hydrochloric acid. The neutralization
reaction is:
NaOH(aq) + HCl(aq) +H₂O(l) + NaCl(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning
