17. Which reaction will not occur as suggested? vloeih Al (s) + SnCh(aq)→AIC13(aq) + Sn(s) b. H2(g) + AgNO3(aq) → Mg(s) + CuSO4(aq) d. Mg(s)+ CaSO((aq) - Ba (s) + HCl(aq) BaCl2(aq) + H2(g) a. -> HNO;(aq) + Ag(s) → Cu(s) + MgSO4(aq) MgSO4(aq) + Ca(s) с. -> e. >
17. Which reaction will not occur as suggested? vloeih Al (s) + SnCh(aq)→AIC13(aq) + Sn(s) b. H2(g) + AgNO3(aq) → Mg(s) + CuSO4(aq) d. Mg(s)+ CaSO((aq) - Ba (s) + HCl(aq) BaCl2(aq) + H2(g) a. -> HNO;(aq) + Ag(s) → Cu(s) + MgSO4(aq) MgSO4(aq) + Ca(s) с. -> e. >
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 99CWP: The following reaction occurs in pure water: H2O(l)+H2O(l)H3O+(aq)+OH-(aq) which is often...
Related questions
Question
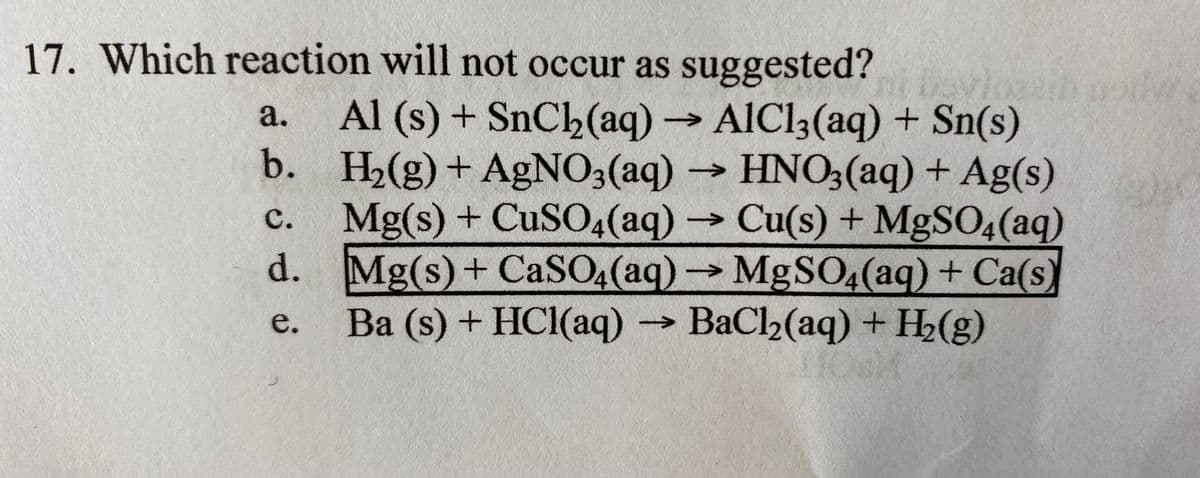
Transcribed Image Text:17. Which reaction will not occur as suggested?
Al (s) + SnCh(aq)AIC13(aq) + Sn(s)
b. H2(g)+ AGNO3(aq) → HNO3(aq) + Ag(s)
Mg(s) + CUSO4(aq) Cu(s) + MgSO4(aq)
d. Mg(s)+ CaSO4(aq)MgSO4(aq) + Ca(s)
Ba (s) + HCl(aq) BaCl2(aq) + H2(g)
а.
с.
->
->
e.
->
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning