Chapter10: Radioactivity And Nuclear Processes
Section: Chapter Questions
Problem 10.19E
Related questions
Question
11
Please answer in reagular notation
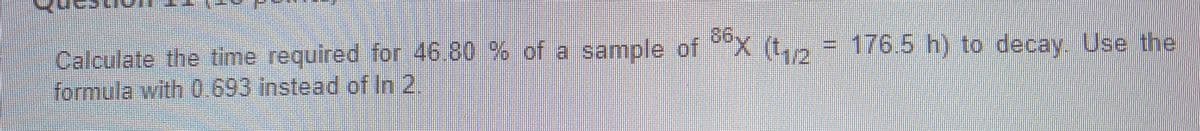
Transcribed Image Text:86x (12
=D176.5h) to decay. Use the
Calculate the time required for 46.80 % of a sample of
formula with 0.693 instead of In 2.
Expert Solution
Step 1
Given : Half life i.e t1/2 of reactant (radioactive isotope) = 176.5 hr.
And percent of reactant reacted 46.80 %.
Step 2
Since the decay reactions are first order reactions.
And we know that the relationship between the amount of reactant i.e [A] and time for a first order reaction is given by
=> ln
where [A] = final amount of reactant = (1 - 0.4680) [A]0 = 0.532 [A]0 (because 46.8 % is decaying)
[A]0 = initial amount of reactant = [A]0
t = time
K = Rate constant
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning