Chapter15: Complex Acid/base Systems
Section: Chapter Questions
Problem 15.4QAP
Related questions
Question
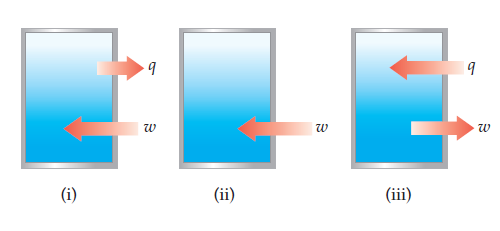
The contents of the closed box in each of the following illustrations
represent a system, and the arrows show the
changes to the system during some process. The lengths
of the arrows represent the relative magnitudes of q and w.
(a) Which of these processes is endothermic? (b) For which
of these processes, if any, is ΔE < 0? (c) For which process,
if any, does the system experience a net gain in internal energy?
Transcribed Image Text:19
(i)
(ii)
(iii)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

