19. If the pressure of a gas is increased and its volume remains constant, what will happen to its temperature? a. The temperature increases. b. The temperature decreases. c. The temperature remains the same. d. The temperature becomes zero. 20. What are the conditions for standard temperature and pressure (STP)? a. 0 °C. 1 atm C. -273.15 °C. 1 atm b. 0 K, 1 atm d. 273.15 K, 10 atm
19. If the pressure of a gas is increased and its volume remains constant, what will happen to its temperature? a. The temperature increases. b. The temperature decreases. c. The temperature remains the same. d. The temperature becomes zero. 20. What are the conditions for standard temperature and pressure (STP)? a. 0 °C. 1 atm C. -273.15 °C. 1 atm b. 0 K, 1 atm d. 273.15 K, 10 atm
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
Section: Chapter Questions
Problem 8STP
Related questions
Question
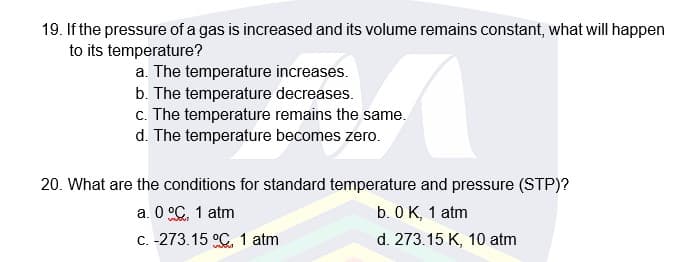
Transcribed Image Text:19. If the pressure of a gas is increased and its volume remains constant, what will happen
to its temperature?
a. The temperature increases.
b. The temperature decreases.
c. The temperature remains the same.
d. The temperature becomes zero.
20. What are the conditions for standard temperature and pressure (STP)?
a. 0 °C. 1 atm
C. -273.15 °C. 1 atm
b. 0 K, 1 atm
d. 273.15 K, 10 atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning