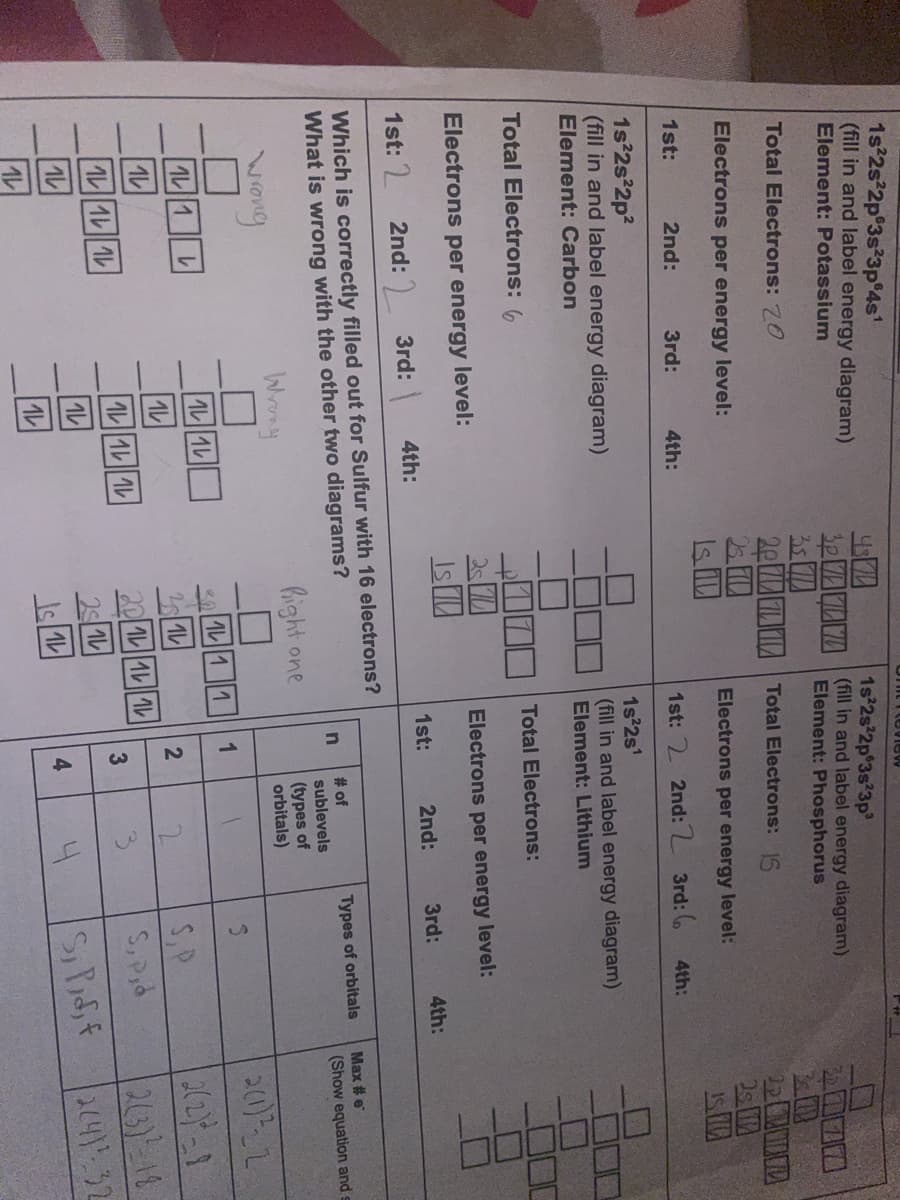
1s 2s 2p°3s 3p 4s (fill in and label energy diagram) Element: Potassium 1s 2s 2p 3s 3p 30 VALYA (ill in and label energy diagram) Element: Phosphorus 2010 Total Electrons: 20 20 LI Total Electrons: IS Electrons per energy level: Electrons per energy level: 1st: 2nd: 3rd: 4th: 1st: 2 2nd:2 3rd: 6 4th: 1s 2s 2p? (fill in and label energy diagram) Element: Carbon 1s 2s' (fill in and label energy diagram) Element: Lithium Total Electrons: 6 Total Electrons: 25 IsI Electrons per energy level: Electrons per energy level: 1st: 2 2nd: ) 3rd: 4th: 1st: 2nd: 3rd: 4th: Which is correctly filled out for Sulfur with 16 electrons? What is wrong with the other two diagrams? Max # e # of sublevels Types of orbitals (Show equation and Wrony hight one (types of orbitals) 20)2 |レ||レ 30 1L11 1L1 1. S.P S, Pid 1L |レ|レ|| ル 20 1L1 1 263)-18 ル|レ|| ル 3. 4. A14132 4.
1s 2s 2p°3s 3p 4s (fill in and label energy diagram) Element: Potassium 1s 2s 2p 3s 3p 30 VALYA (ill in and label energy diagram) Element: Phosphorus 2010 Total Electrons: 20 20 LI Total Electrons: IS Electrons per energy level: Electrons per energy level: 1st: 2nd: 3rd: 4th: 1st: 2 2nd:2 3rd: 6 4th: 1s 2s 2p? (fill in and label energy diagram) Element: Carbon 1s 2s' (fill in and label energy diagram) Element: Lithium Total Electrons: 6 Total Electrons: 25 IsI Electrons per energy level: Electrons per energy level: 1st: 2 2nd: ) 3rd: 4th: 1st: 2nd: 3rd: 4th: Which is correctly filled out for Sulfur with 16 electrons? What is wrong with the other two diagrams? Max # e # of sublevels Types of orbitals (Show equation and Wrony hight one (types of orbitals) 20)2 |レ||レ 30 1L11 1L1 1. S.P S, Pid 1L |レ|レ|| ル 20 1L1 1 263)-18 ル|レ|| ル 3. 4. A14132 4.
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 4STP
Related questions
Question
Transcribed Image Text:ell
1s 2s 2p°3s 3p 4s
(fill in and label energy diagram)
1s 2s 2p 3s 3p
3P LNA (fill in and label energy diagram)
Element: Phosphorus
Element: Potassium
Total Electrons: 20
35
20 LI Total Electrons: IS
22 A
Electrons per energy level:
Electrons per energy level:
1st:
2nd:
3rd:
4th:
1st: 2 2nd:2 3rd: 6
4th:
1s 2s 2p?
(fill in and label energy diagram)
Element: Carbon
1s 2s'
(fill in and label energy diagram)
Element: Lithium
Total Electrons: 6
Total Electrons:
2 ID
Is
Electrons per energy level:
Electrons per energy level:
1st: 2
2nd: ) 3rd:
4th:
1st:
2nd:
Зrd:
4th:
Which is correctly filled out for Sulfur with 16 electrons?
What is wrong with the other two diagrams?
Max # e
# of
sublevels
Types of orbitals
n
(Show equation and
Wrny
Pight one
(types of
orbitals)
|レ||1レ
1L11
30
20 11 1
3
23)-18
214) 32
L1L 1
|レ|レ|| ル
3.
S,Pid
2516
4
4.
1L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning



General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning