an empty The complete electron configuration of Mg is 1s 2s 2p 3s whereas the complete electron configuration of Al is more 3s Removing an electron from the orbital of Mg 3p requires energy than removing an electron from the orbital of Al. 1s°2s²2p®3s°3p' a partially filled However, when adding an electron, the electron affinity for Mg is negative than that of Al because the electron is a completely filled being added to sublevel. 1s 2s 2p°3s less 1s2s 2p°3s²3p²
an empty The complete electron configuration of Mg is 1s 2s 2p 3s whereas the complete electron configuration of Al is more 3s Removing an electron from the orbital of Mg 3p requires energy than removing an electron from the orbital of Al. 1s°2s²2p®3s°3p' a partially filled However, when adding an electron, the electron affinity for Mg is negative than that of Al because the electron is a completely filled being added to sublevel. 1s 2s 2p°3s less 1s2s 2p°3s²3p²
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter7: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 180CP: Answer the following questions, assuming that ms, could have three values rather than two and that...
Related questions
Question
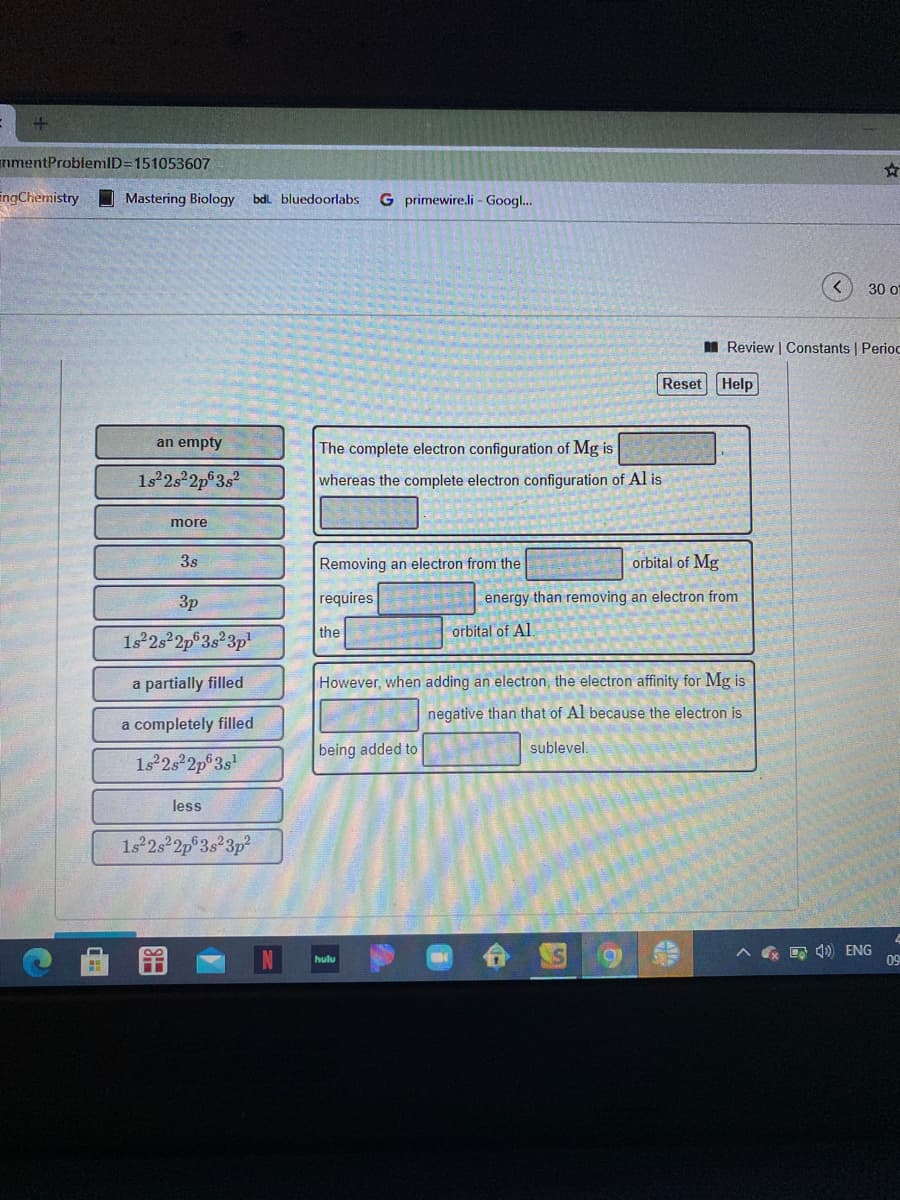
Transcribed Image Text:an empty
The complete electron configuration of Mg is
1s 2s 2p 3s
whereas the complete electron configuration of Al is
more
3s
Removing an electron from the
orbital of Mg
3p
requires
energy than removing an electron from
the
orbital of Al.
1s°2s²2p®3s°3p'
a partially filled
However, when adding an electron, the electron affinity for Mg is
negative than that of Al because the electron is
a completely filled
being added to
sublevel.
1s 2s 2p°3s
less
1s2s 2p°3s²3p²
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
