an electron to a neutral gaseous atom and is related to an element's position on the periodic table. Electron affinities can be positive, negative, or zero, as shown in the table. Part A Electron affinity (kJ/mol) For the elements with the electron affinities given in the table in the introduction, which element is most likely to accept an electron? Element As (arsenic) -78 • View Available Hint(s) F (fluorine) -328 Mg (magnesium) > 0 O As O F O Mg
an electron to a neutral gaseous atom and is related to an element's position on the periodic table. Electron affinities can be positive, negative, or zero, as shown in the table. Part A Electron affinity (kJ/mol) For the elements with the electron affinities given in the table in the introduction, which element is most likely to accept an electron? Element As (arsenic) -78 • View Available Hint(s) F (fluorine) -328 Mg (magnesium) > 0 O As O F O Mg
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter2: Atomic Structure And Periodicity
Section: Chapter Questions
Problem 11ALQ: Consider the following statement "The ionization energy for the potassium atom is negative, because...
Related questions
Question
Please answer question 18 part A and B
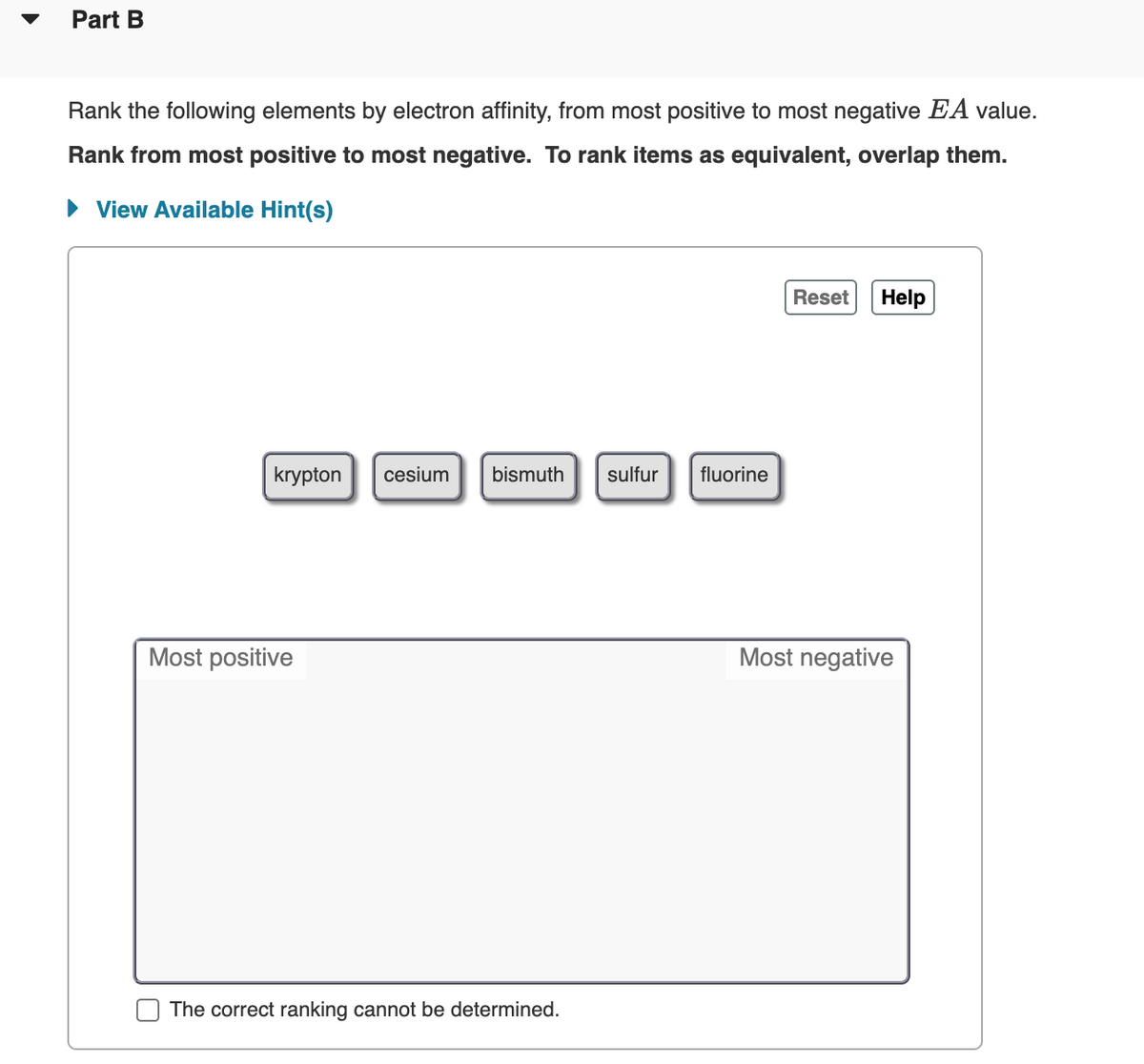
Transcribed Image Text:Part B
Rank the following elements by electron affinity, from most positive to most negative EA value.
Rank from most positive to most negative. To rank items as equivalent, overlap them.
• View Available Hint(s)
Reset
Help
krypton
cesium
bismuth
sulfur
fluorine
Most positive
Most negative
The correct ranking cannot be determined.
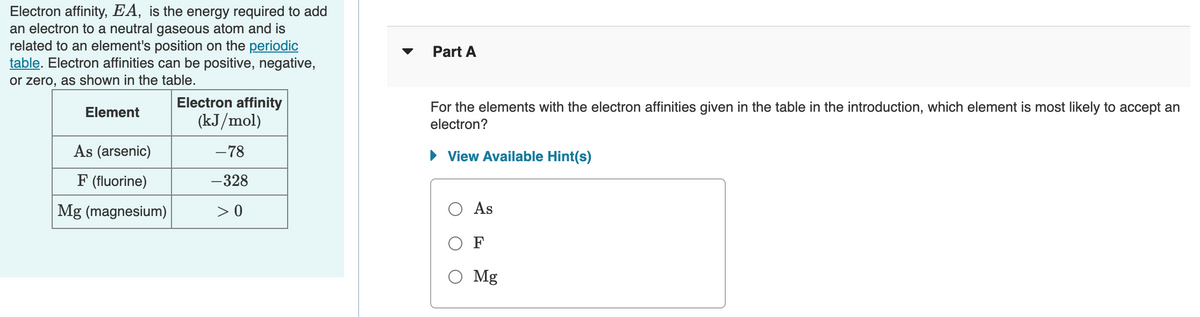
Transcribed Image Text:Electron affinity, EA, is the energy required to add
an electron to a neutral gaseous atom and is
related to an element's position on the periodic
table. Electron affinities can be positive, negative,
or zero, as shown in the table.
Part A
Electron affinity
For the elements with the electron affinities given in the table in the introduction, which element is most likely to accept an
electron?
Element
(kJ/mol)
As (arsenic)
-78
• View Available Hint(s)
F (fluorine)
-328
Mg (magnesium)
> 0
O As
O F
O Mg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning