Aluminum Iron 7.5% Other 4.7% 9.2% Calcium 3.4% Silicon 25.7% Oxygen 49.5% Earth's crust Other 7% Hydrogen 10% Oxygen 65% Carbon 18% Human body Figure 1.6 Relative abundances of elements.* Elements in percent by mass in Earth's crust (including oceans and atmosphere) and the human body.
Aluminum Iron 7.5% Other 4.7% 9.2% Calcium 3.4% Silicon 25.7% Oxygen 49.5% Earth's crust Other 7% Hydrogen 10% Oxygen 65% Carbon 18% Human body Figure 1.6 Relative abundances of elements.* Elements in percent by mass in Earth's crust (including oceans and atmosphere) and the human body.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 59QRT
Related questions
Question
If the lower pie chart was drawn as
the percentage in terms of number of
atoms rather than the percentage in
terms of mass, would the hydrogen
slice of the pie get larger or smaller?
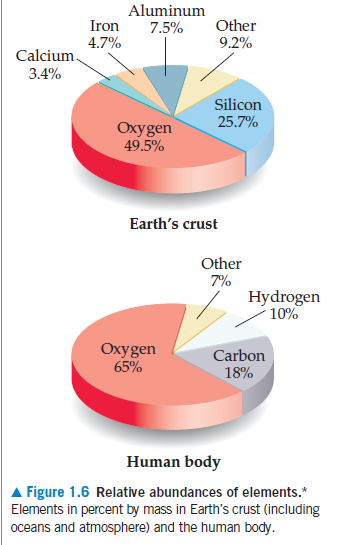
Transcribed Image Text:Aluminum
Iron
7.5%
Other
4.7%
9.2%
Calcium
3.4%
Silicon
25.7%
Oxygen
49.5%
Earth's crust
Other
7%
Hydrogen
10%
Oxygen
65%
Carbon
18%
Human body
Figure 1.6 Relative abundances of elements.*
Elements in percent by mass in Earth's crust (including
oceans and atmosphere) and the human body.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER