Electroplating is rarely 100% efficient-some of the current usually is consumed by reactions other than the desired reduction reaction. The current efficiency is the fraction of charge that does go into the desired reduction reaction. When a current of 6.00 A flows for 15.0 minutes through a cell composed of two Pt electrodes in a solution of Cu(NO3)2(aq) in dilute HNO3(aq), 0.40 g deposited on the cathode. of copper is (a) Calculate the current efficiency for this process. 22.5 % (b) If the remainder of the current reduces equal amounts of hydronium (to hydrogen gas and water) and nitrate ions (to NO(g)), calculate the volumes of H2(g) and NO(g) generated (in L) per hour at 1 bar and 30°C. L H2(g) L NO(g)
Electroplating is rarely 100% efficient-some of the current usually is consumed by reactions other than the desired reduction reaction. The current efficiency is the fraction of charge that does go into the desired reduction reaction. When a current of 6.00 A flows for 15.0 minutes through a cell composed of two Pt electrodes in a solution of Cu(NO3)2(aq) in dilute HNO3(aq), 0.40 g deposited on the cathode. of copper is (a) Calculate the current efficiency for this process. 22.5 % (b) If the remainder of the current reduces equal amounts of hydronium (to hydrogen gas and water) and nitrate ions (to NO(g)), calculate the volumes of H2(g) and NO(g) generated (in L) per hour at 1 bar and 30°C. L H2(g) L NO(g)
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.116QP
Related questions
Question
100%
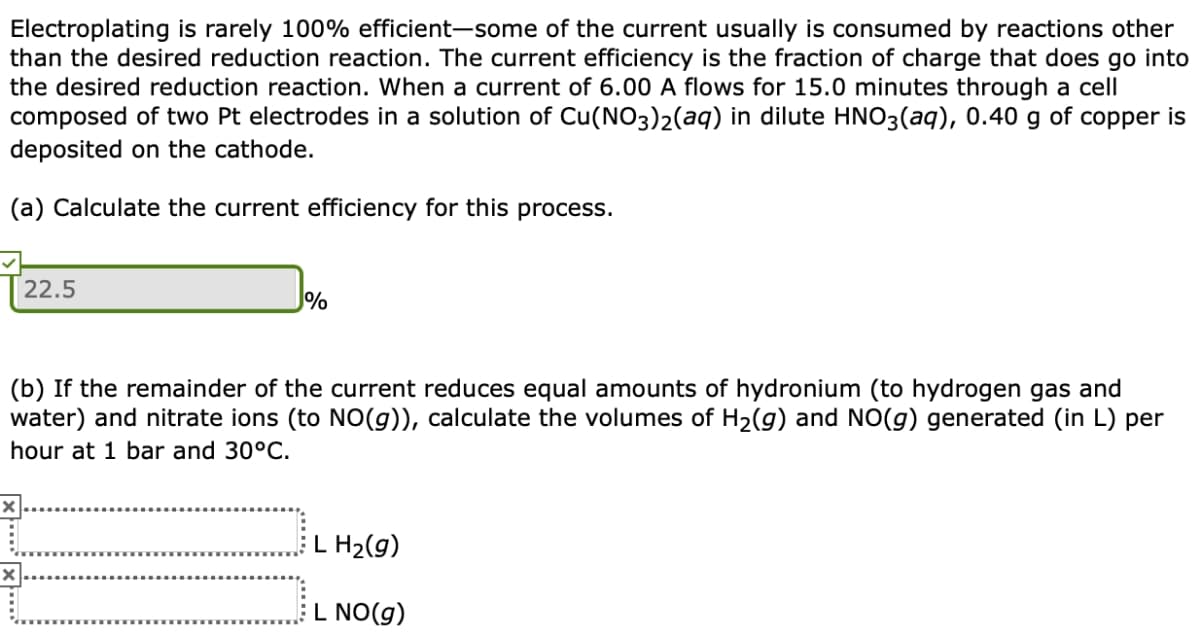
Transcribed Image Text:Electroplating is rarely 100% efficient-some of the current usually is consumed by reactions other
than the desired reduction reaction. The current efficiency is the fraction of charge that does go into
the desired reduction reaction. When a current of 6.00 A flows for 15.0 minutes through a cell
composed of two Pt electrodes in a solution of Cu(NO3)2(aq) in dilute HNO3(aq), 0.40 g of copper is
deposited on the cathode.
(a) Calculate the current efficiency for this process.
22.5
%
(b) If the remainder of the current reduces equal amounts of hydronium (to hydrogen gas and
water) and nitrate ions (to NO(g)), calculate the volumes of H2(g) and NO(g) generated (in L) per
hour at 1 bar and 30°C.
L H2(g)
L NO(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning