The gram quantities of both reactants, C2H6 and O2are given for the following balanced reaction. Mass of the CO2 that was (actually) measured at the end of the experiment is also given in parenthesis next to CO2. (38.0 g). Complete the missing values (i), (ii), ........ (xiv) and answer the following questions. (Copy the table and paste to answer box, or just write, i, ii, ii.. and give the answer next to that. Clue: Find the limiting reactatnt to get the theoretical amount of CO2) 2 C2H6 + 7 02 6 H20 + 4 CO2 (38g) Mass (g) 15.0 64.0 i? ii? Molar Mass (g/mol) iii? iv? v? vi? Moles reacted or produced vii? viii? ix? x? Mole ratio xi? xii? xiii? xiv? a) What is the limiting reactant of this reaction? b) What is the percent yield of CO2?
The gram quantities of both reactants, C2H6 and O2are given for the following balanced reaction. Mass of the CO2 that was (actually) measured at the end of the experiment is also given in parenthesis next to CO2. (38.0 g). Complete the missing values (i), (ii), ........ (xiv) and answer the following questions. (Copy the table and paste to answer box, or just write, i, ii, ii.. and give the answer next to that. Clue: Find the limiting reactatnt to get the theoretical amount of CO2) 2 C2H6 + 7 02 6 H20 + 4 CO2 (38g) Mass (g) 15.0 64.0 i? ii? Molar Mass (g/mol) iii? iv? v? vi? Moles reacted or produced vii? viii? ix? x? Mole ratio xi? xii? xiii? xiv? a) What is the limiting reactant of this reaction? b) What is the percent yield of CO2?
ChapterU6: Showtime: Reversible Reactions And Chemical Equilibrium
Section: Chapter Questions
Problem 7STP
Related questions
Question
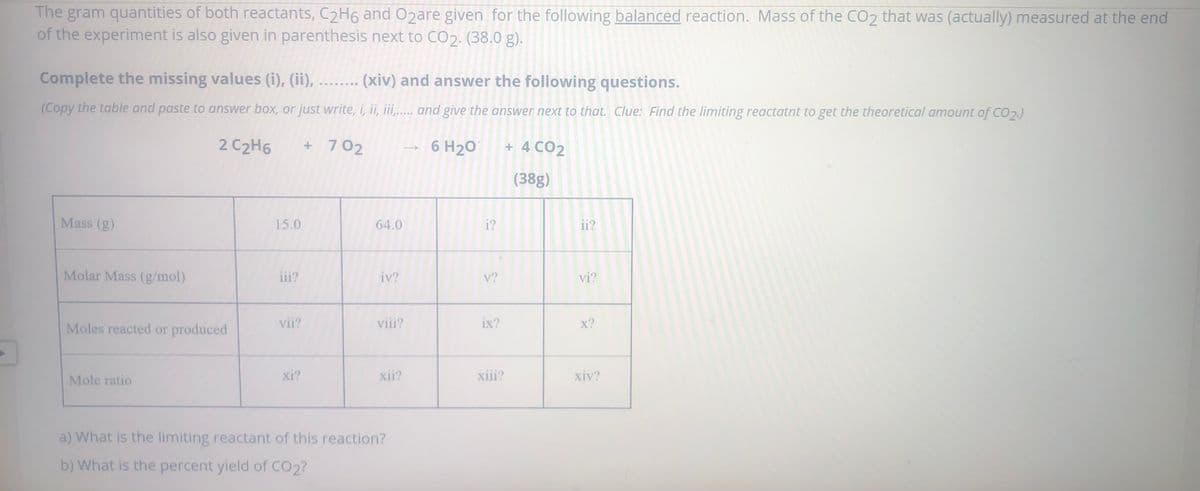
Transcribed Image Text:The gram quantities of both reactants, C2H6 and O2are given for the following balanced reaction. Mass of the CO2 that was (actually) measured at the end
of the experiment is also given in parenthesis next to CO2. (38.0 g).
Complete the missing values (i), (ii),
(xiv) and answer the following questions.
(Copy the table and paste to answer box, or just write, i, ii, iii,..... and give the answer next to that. Clue: Find the limiting reactatnt to get the theoretical amount of CO2.)
2 C2H6
+ 702
6 H20
+ 4 CO2
(38g)
Mass (g)
15.0
64.0
i?
ii?
Molar Mass (g/mol)
iii?
iv?
v?
vi?
Moles reacted or produced
vii?
viii?
ix?
x?
Mole ratio
xi?
xii?
xiii?
xiv?
a) What is the limiting reactant of this reaction?
b) What is the percent yield of CO2?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

