2. A similar experiment is performed to determine the empirical formula of an oxide of copper, and the following data were collected. Predict the empirical formula of the copper oxide from these data. Mass of crucible, cover, and copper sample Mass of empty crucible with cover 21.53 g 19.66 g Mass of crucible and cover and sample (after heating) 21.76 g How can the experiment for the determination of the empirical formula of an oxide of copper be improved?
2. A similar experiment is performed to determine the empirical formula of an oxide of copper, and the following data were collected. Predict the empirical formula of the copper oxide from these data. Mass of crucible, cover, and copper sample Mass of empty crucible with cover 21.53 g 19.66 g Mass of crucible and cover and sample (after heating) 21.76 g How can the experiment for the determination of the empirical formula of an oxide of copper be improved?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 14P
Related questions
Question
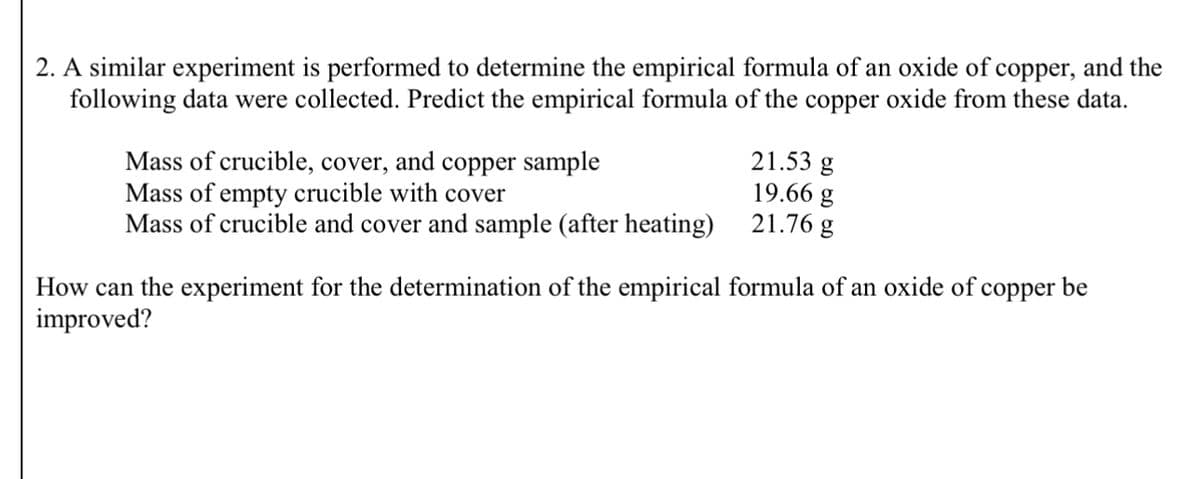
Transcribed Image Text:2. A similar experiment is performed to determine the empirical formula of an oxide of copper, and the
following data were collected. Predict the empirical formula of the copper oxide from these data.
Mass of crucible, cover, and copper sample
Mass of empty crucible with cover
21.53 g
19.66 g
Mass of crucible and cover and sample (after heating)
21.76 g
How can the experiment for the determination of the empirical formula of an oxide of copper be
improved?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning