2. a. Rank molecules A-D (1 = lowest, 4 = highest) in order of increasing boiling %3D point. Briefly rationalize your ranking. NH2 HN A в D b. How many degrees of unsaturation do each of these molecules have? c. Label each amine as primary, secondary, or tertiary.
2. a. Rank molecules A-D (1 = lowest, 4 = highest) in order of increasing boiling %3D point. Briefly rationalize your ranking. NH2 HN A в D b. How many degrees of unsaturation do each of these molecules have? c. Label each amine as primary, secondary, or tertiary.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterNW4: Nomenclature Worksheet 4: Carbonyl Compounds
Section: Chapter Questions
Problem 14CTQ
Related questions
Question
Rank molecules A-D (1=lowest, 4=highest) in order of increasing boiling point. Briefly rationalize your ranking. Please answer all parts.
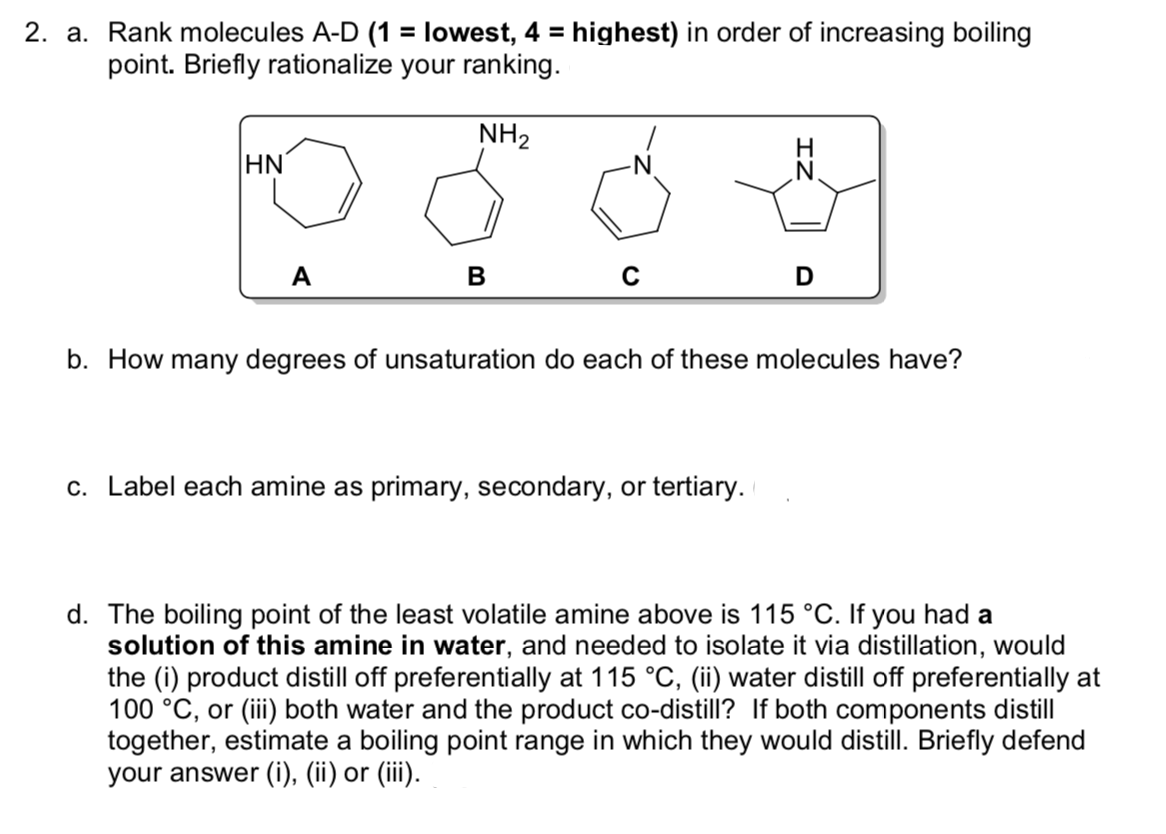
Transcribed Image Text:2. a. Rank molecules A-D (1 = lowest, 4 = highest) in order of increasing boiling
point. Briefly rationalize your ranking.
NH2
H.
HN
-N
A
в
b. How many degrees of unsaturation do each of these molecules have?
c. Label each amine as primary, secondary, or tertiary.
d. The boiling point of the least volatile amine above is 115 °C. If you had a
solution of this amine in water, and needed to isolate it via distillation, would
the (i) product distill off preferentially at 115 °C, (ii) water distill off preferentially at
100 °C, or (iii) both water and the product co-distill? If both components distill
together, estimate a boiling point range in which they would distill. Briefly defend
your answer (i), (ii) or (iii).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning