2. Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g)= 2NH3(g) equilibrium constant (Kp values) as a function of temperature are 300 °С, 4.34 х 10-3 500 °С, 1.45 х 10-5 600 °С, 2.25 х 10-6 (a) Is the reaction exothermic or endothermic? Explain why. (b) Calculate AH° from the data using the van't Hoff equation: (K2 In ΔΗ rxn T2. Is the sign of AH° in your answer consistent with your answer for (a)?
2. Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g)= 2NH3(g) equilibrium constant (Kp values) as a function of temperature are 300 °С, 4.34 х 10-3 500 °С, 1.45 х 10-5 600 °С, 2.25 х 10-6 (a) Is the reaction exothermic or endothermic? Explain why. (b) Calculate AH° from the data using the van't Hoff equation: (K2 In ΔΗ rxn T2. Is the sign of AH° in your answer consistent with your answer for (a)?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.118PAE
Related questions
Question
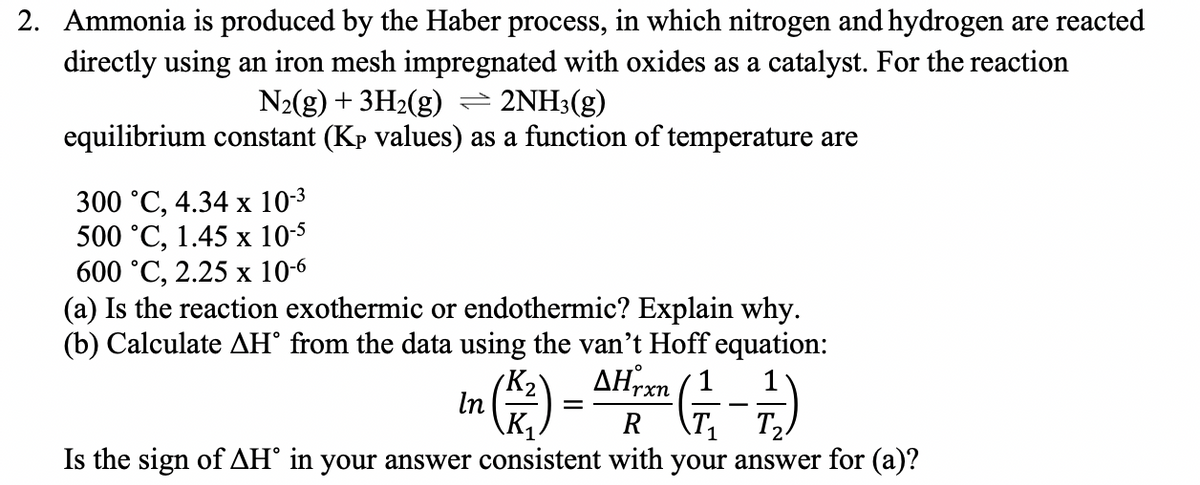
Transcribed Image Text:2. Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted
directly using an iron mesh impregnated with oxides as a catalyst. For the reaction
N2(g) + 3H2(g)= 2NH3(g)
equilibrium constant (Kp values) as a function of temperature are
300 °С, 4.34 х 10-3
500 °C, 1.45 х 10-5
600 °C, 2.25 х 10-6
(a) Is the reaction exothermic or endothermic? Explain why.
(b) Calculate AH° from the data using the van't Hoff equation:
AHrxn
K2
In
\K.
R
\71
T2
Is the sign of AH° in your answer consistent with your answer for (a)?
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning