2. Assume that a given solution contains Ag+ or Ba2+ or a mixture of both. A reasonable method for doing a qualitative analysis on this solution might be sketched out as follows (Remember || means a precipitate forms and | means a solution remains. It is always assumed that solutions are separated from precipitates before adding the next reagent.) Solution containing Ag* and Ba2+ Na,SO4 a. What is the precipitate formed at X in the above scheme? ER b. What is the identity of cation at Y in the above scheme? c. What are two other solutions that could be used in the above scheme rather than Na,SO,? Explain
2. Assume that a given solution contains Ag+ or Ba2+ or a mixture of both. A reasonable method for doing a qualitative analysis on this solution might be sketched out as follows (Remember || means a precipitate forms and | means a solution remains. It is always assumed that solutions are separated from precipitates before adding the next reagent.) Solution containing Ag* and Ba2+ Na,SO4 a. What is the precipitate formed at X in the above scheme? ER b. What is the identity of cation at Y in the above scheme? c. What are two other solutions that could be used in the above scheme rather than Na,SO,? Explain
Chapter8: Polyfunctional Acids And Bases
Section: Chapter Questions
Problem 9P
Related questions
Question
all 3 parts please
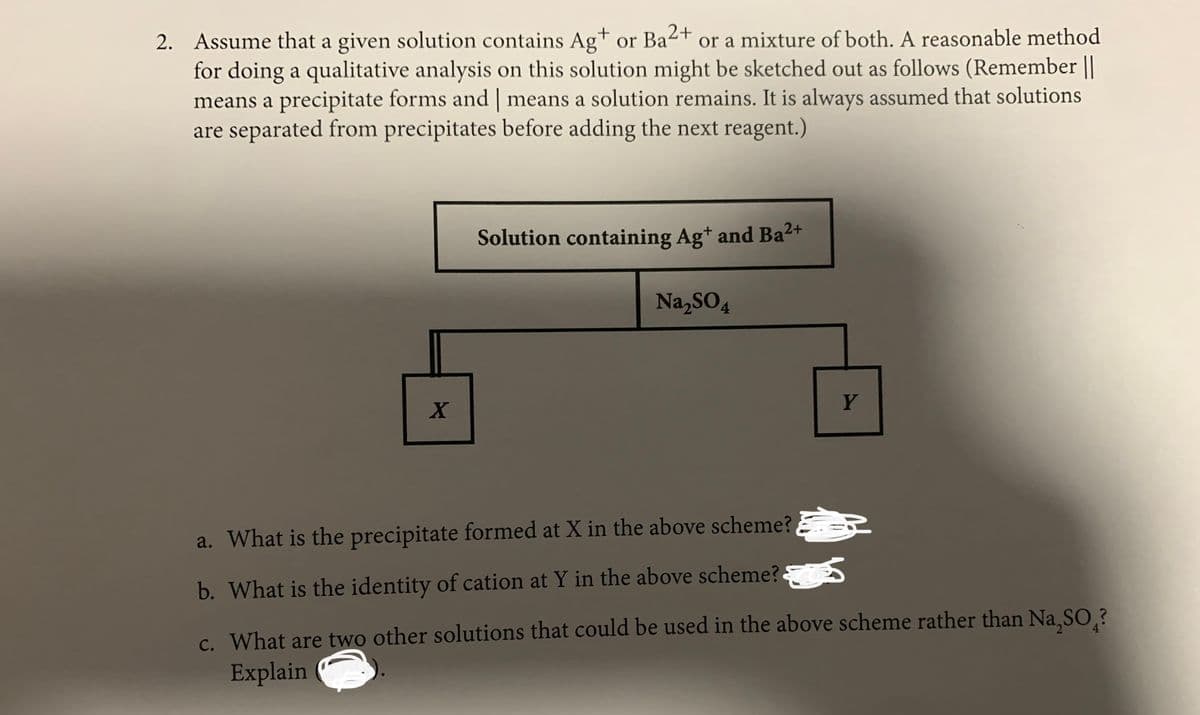
Transcribed Image Text:2. Assume that a given solution contains Ag+ or Ba2+
for doing a qualitative analysis on this solution might be sketched out as follows (Remember ||
means a precipitate forms and | means a solution remains. It is always assumed that solutions
are separated from precipitates before adding the next reagent.)
or a mixture of both. A reasonable method
Solution containing Ag* and Ba2+
Na,SO4
Y
a. What is the precipitate formed at X in the above scheme? ER
b. What is the identity of cation at Y in the above scheme?
c. What are two other solutions that could be used in the above scheme rather than Na SO.?
Explain
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning