2. Consider the reaction: 2 NH3 + 5 F2 → N2F4 6 HF a) How many grams of N2F, can be formed from 4.0 grams of NH3 and 14.0 grams of F,? b) What is the limiting reactant in the reaction? c) How many grams of HF is formed? d) How much of excess reactant will be left over from the reaction? e) If 4.8 grams of N„F, is obtained in the reaction, what is the percent yield of this reaction? Molar mass NH, - 17.04 g/mol Molar mass N2F, - 104.02 g/mol Molar mass F2 38 g/mol - 20.01 g/mol Molar mass HF
2. Consider the reaction: 2 NH3 + 5 F2 → N2F4 6 HF a) How many grams of N2F, can be formed from 4.0 grams of NH3 and 14.0 grams of F,? b) What is the limiting reactant in the reaction? c) How many grams of HF is formed? d) How much of excess reactant will be left over from the reaction? e) If 4.8 grams of N„F, is obtained in the reaction, what is the percent yield of this reaction? Molar mass NH, - 17.04 g/mol Molar mass N2F, - 104.02 g/mol Molar mass F2 38 g/mol - 20.01 g/mol Molar mass HF
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU4: Toxins: Stoichiometry, Solution Chemistry, And Acids And Bases
SectionU4.25: Mole Tunnel: Stoichiometry
Problem 3E
Related questions
Question
I need an answer for item no. 2 specifically on letter d and e.
I need the correct answer, please with correct solution. Thank you.
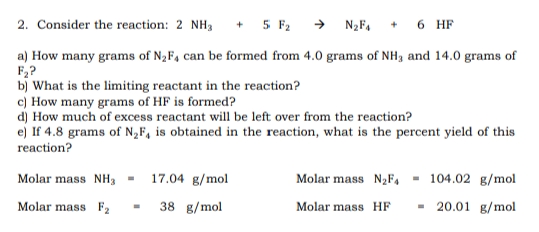
Transcribed Image Text:2. Consider the reaction: 2 NH3
+ 5 F2 → N2F,
6 HF
a) How many grams of N2F4 can be formed from 4.0 grams of NH3 and 14.0 grams of
F,?
b) What is the limiting reactant in the reaction?
c) How many grams of HF is formed?
d) How much of excess reactant will be left over from the reaction?
e) If 4.8 grams of N„F, is obtained in the reaction, what is the percent yield of this
reaction?
Molar mass NH, -
17.04 g/mol
Molar mass N2F, - 104.02 g/mol
Molar mass F2
38 g/mol
- 20.01 g/mol
Molar mass HF
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
