2. Explain the following observations: (a) Co(III) forms strong bonds to O- and N-donor ligands, moderately strong bonds to P-donor ligand, and weak bonds to As-donor ligands. (b) EDTA4 forms more stable complexes with trivalent first-row transition metals (M3+) than it does with the corresponding divalent metal (M²+) of each (e.g. Cr²+ vs Cr3+). EDTA is a common ligand which bonds a metal center through four oxygen and two nitrogen atoms in an octahedral geometry (Figure 1). Figure 1. EDTA binds in an octahedral geometry
2. Explain the following observations: (a) Co(III) forms strong bonds to O- and N-donor ligands, moderately strong bonds to P-donor ligand, and weak bonds to As-donor ligands. (b) EDTA4 forms more stable complexes with trivalent first-row transition metals (M3+) than it does with the corresponding divalent metal (M²+) of each (e.g. Cr²+ vs Cr3+). EDTA is a common ligand which bonds a metal center through four oxygen and two nitrogen atoms in an octahedral geometry (Figure 1). Figure 1. EDTA binds in an octahedral geometry
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter14: Applications Of Ultraviolet-visible Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 14.7QAP: A 3.03-g petroleum specimen was decomposed by wet ashing and subsequently diluted to 500 mL in a...
Related questions
Question
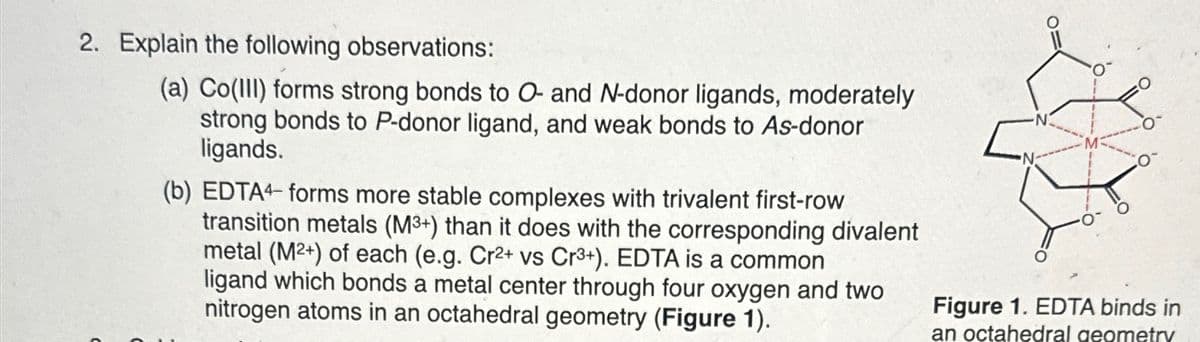
Transcribed Image Text:2. Explain the following observations:
(a) Co(III) forms strong bonds to O- and N-donor ligands, moderately
strong bonds to P-donor ligand, and weak bonds to As-donor
ligands.
(b) EDTA4 forms more stable complexes with trivalent first-row
transition metals (M3+) than it does with the corresponding divalent
metal (M²+) of each (e.g. Cr²+ vs Cr3+). EDTA is a common
ligand which bonds a metal center through four oxygen and two
nitrogen atoms in an octahedral geometry (Figure 1).
Figure 1. EDTA binds in
an octahedral geometry
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning