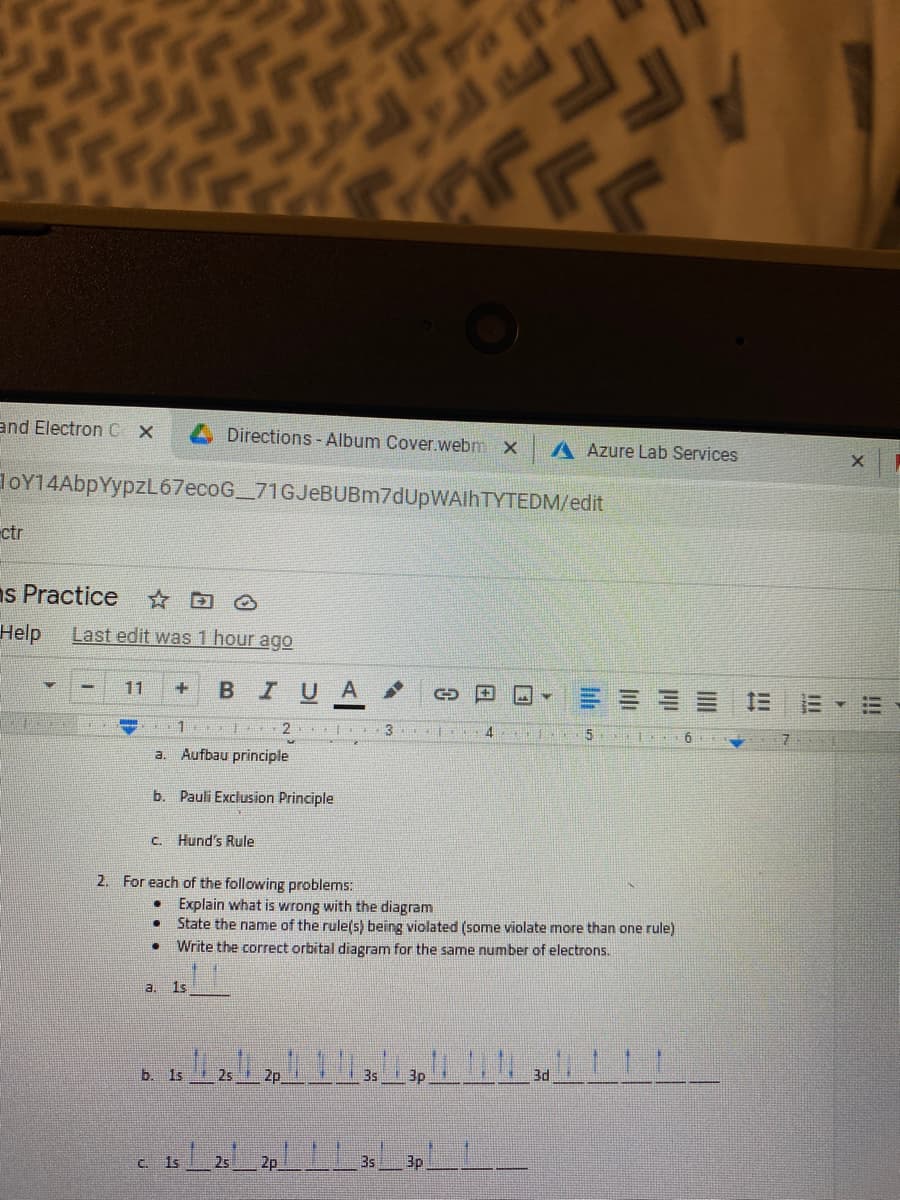
2. For each of the following problems: • Explain what is wrong with the diagram • State the name of the rule(s) being violated (some violate more than one rule) Write the correct orbital diagram for the same number of electrons. a. 1s b. 1s 25 35 3p 3d 20 35 3p C. 1s 25
2. For each of the following problems: • Explain what is wrong with the diagram • State the name of the rule(s) being violated (some violate more than one rule) Write the correct orbital diagram for the same number of electrons. a. 1s b. 1s 25 35 3p 3d 20 35 3p C. 1s 25
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 34PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Question
Can you please help with question 2 really quickly, it’s urgent please. Thank you so much.
Transcribed Image Text:and Electron CX
Directions - Album Cover.webm x
A Azure Lab Services
JOY14AbpYypzL67ecoG 71GJeBUBm7dUpWAlhTYTEDM/edit
ctr
as Practice
Help
Last edit was 1 hour ago
11
BIUA
1 2
3
5
a. Aufbau principle
b. Pauli Exclusion Principle
C. Hund's Rule
2. For each of the following problems:
• Explain what is wrong with the diagram
State the name of the rule(s) being violated (some violate more than one rule)
Write the correct orbital diagram for the same number of electrons.
3. Is
b. 1s
25
3s
3p
3d
C. 1s
!!
Expert Solution
Given
Three set of electronic configurations that violate Aufbau Principle, Hund's rule, Pauli's exclusion principle.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,