Jacksonville University X electron configuration for s Periodic Table /ibiscms/mod/flcn/view.php?id=963 5823 (? HW 3 A 9- RADHAA Activities and Due Dates HW 3 LA GIvelp 00/2600 Resources Hint For the following atoms in their ground state, determine the number of electrons in each energy shell. If there are no electrons in the particular energy shell for an atom, leave it blank. An atom with 6 total electrons. electrons in energy shell 2: electrons in energy shell 1: electrons in energy shell 4: electrons in energy shell 3: An atom with 10 total electrons. electrons in energy shell 2: electrons in energy shell 1: electrons in energy shell 4: electrons in energy shell 3: An atom with 13 total electrons. electrons in energy shell 2: electrons in energy shell 1: electrons in energy shell 4: electrons in energy shell 3: An atom with 20 total electrons. electrons in energy shell 1: electrons in energy shell 2: electrons in energy shell 3: electrons in energy shell 4: about us privacy policy terms of use help contact us careers eN P hp
Jacksonville University X electron configuration for s Periodic Table /ibiscms/mod/flcn/view.php?id=963 5823 (? HW 3 A 9- RADHAA Activities and Due Dates HW 3 LA GIvelp 00/2600 Resources Hint For the following atoms in their ground state, determine the number of electrons in each energy shell. If there are no electrons in the particular energy shell for an atom, leave it blank. An atom with 6 total electrons. electrons in energy shell 2: electrons in energy shell 1: electrons in energy shell 4: electrons in energy shell 3: An atom with 10 total electrons. electrons in energy shell 2: electrons in energy shell 1: electrons in energy shell 4: electrons in energy shell 3: An atom with 13 total electrons. electrons in energy shell 2: electrons in energy shell 1: electrons in energy shell 4: electrons in energy shell 3: An atom with 20 total electrons. electrons in energy shell 1: electrons in energy shell 2: electrons in energy shell 3: electrons in energy shell 4: about us privacy policy terms of use help contact us careers eN P hp
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 36P: Photoelectron spectra were acquired from a sample of gaseous O2 using X-ray radiation with...
Related questions
Question
Transcribed Image Text:Jacksonville University X
electron configuration for s
Periodic Table
/ibiscms/mod/flcn/view.php?id=963 5823
(?
HW 3
A
9- RADHAA
Activities and Due Dates
HW 3
LA GIvelp
00/2600
Resources
Hint
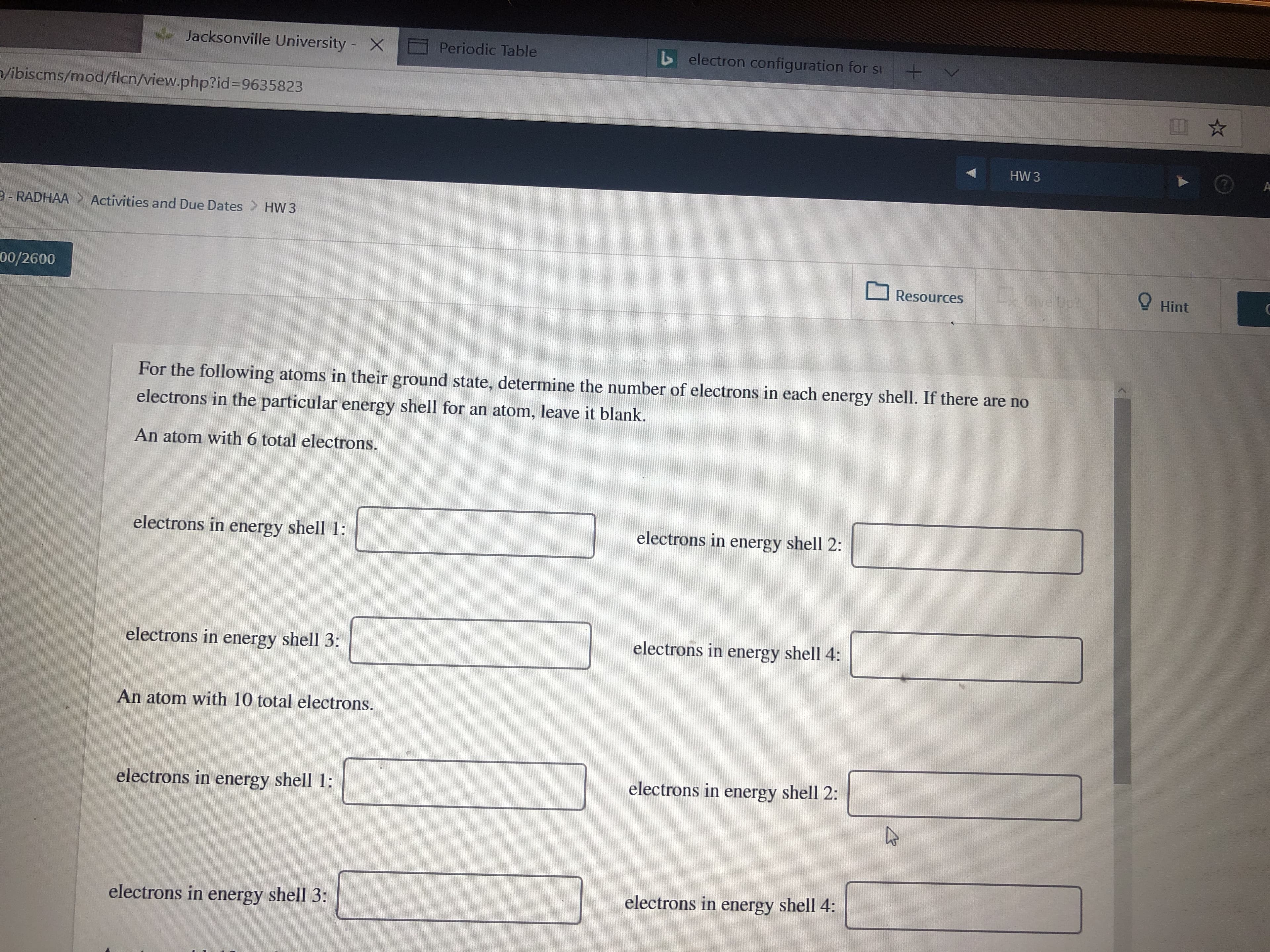
For the following atoms in their ground state, determine the number of electrons in each energy shell. If there are no
electrons in the particular energy shell for an atom, leave it blank.
An atom with 6 total electrons.
electrons in energy shell 2:
electrons in energy shell 1:
electrons in energy shell 4:
electrons in energy shell 3:
An atom with 10 total electrons.
electrons in energy shell 2:
electrons in energy shell 1:
electrons in energy shell 4:
electrons in energy shell 3:
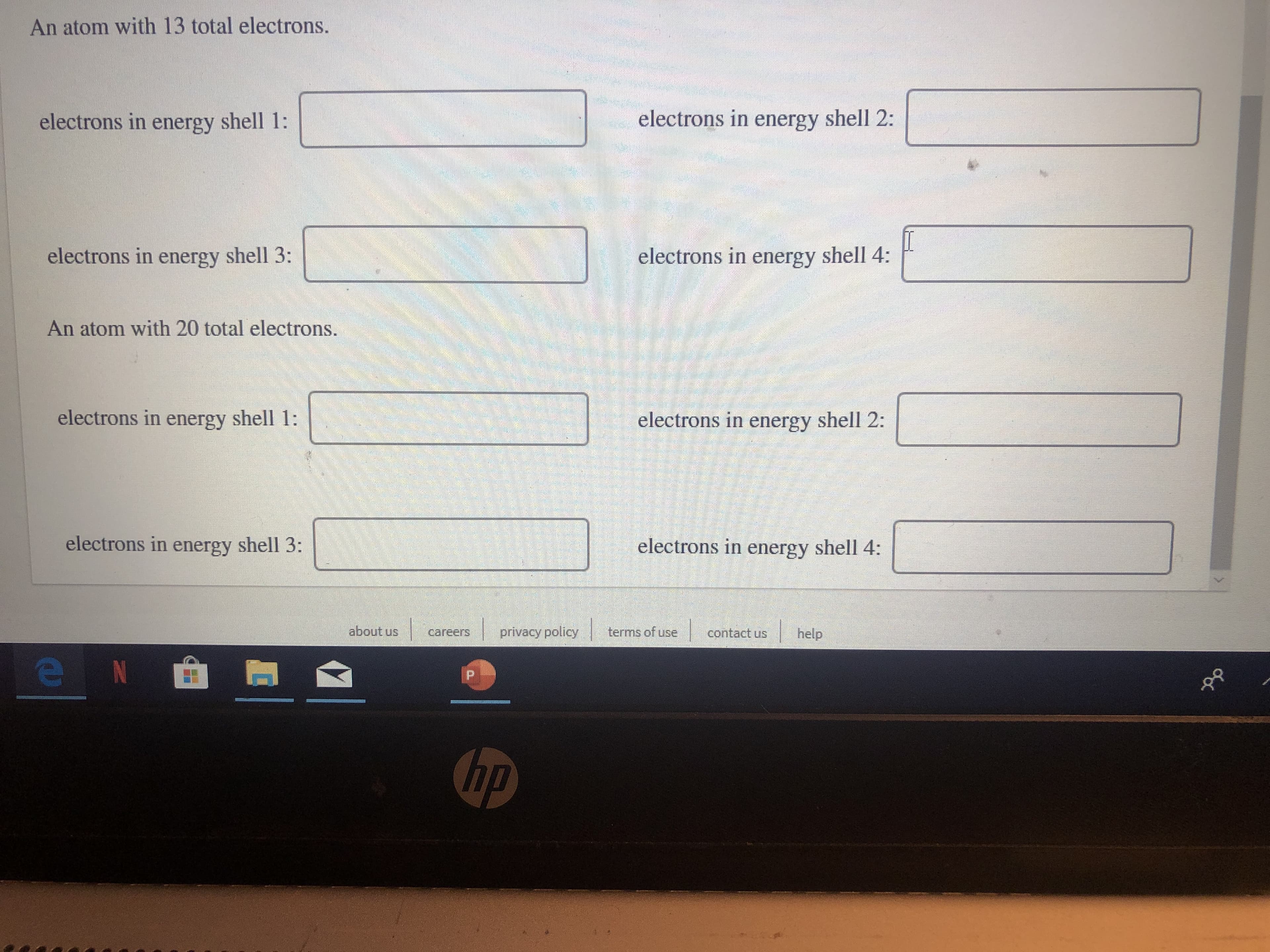
Transcribed Image Text:An atom with 13 total electrons.
electrons in energy shell 2:
electrons in energy shell 1:
electrons in energy shell 4:
electrons in energy shell 3:
An atom with 20 total electrons.
electrons in energy shell 1:
electrons in energy shell 2:
electrons in energy shell 3:
electrons in energy shell 4:
about us
privacy policy
terms of use
help
contact us
careers
eN
P
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,