2. How are gases and liquids similar in properties? * a. Both have molecules that move randomly. b. Both evaporate when sufficient heat is added. C. Both have molecules with very high forces of attraction. d. Both have molecules that held together in a rigid pattern.
2. How are gases and liquids similar in properties? * a. Both have molecules that move randomly. b. Both evaporate when sufficient heat is added. C. Both have molecules with very high forces of attraction. d. Both have molecules that held together in a rigid pattern.
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
SectionU3.5: Absolute Zero: Kelvin Scale
Problem 11E
Related questions
Question
Answer number 2 to 5 pls. I don't want to waste my money here.
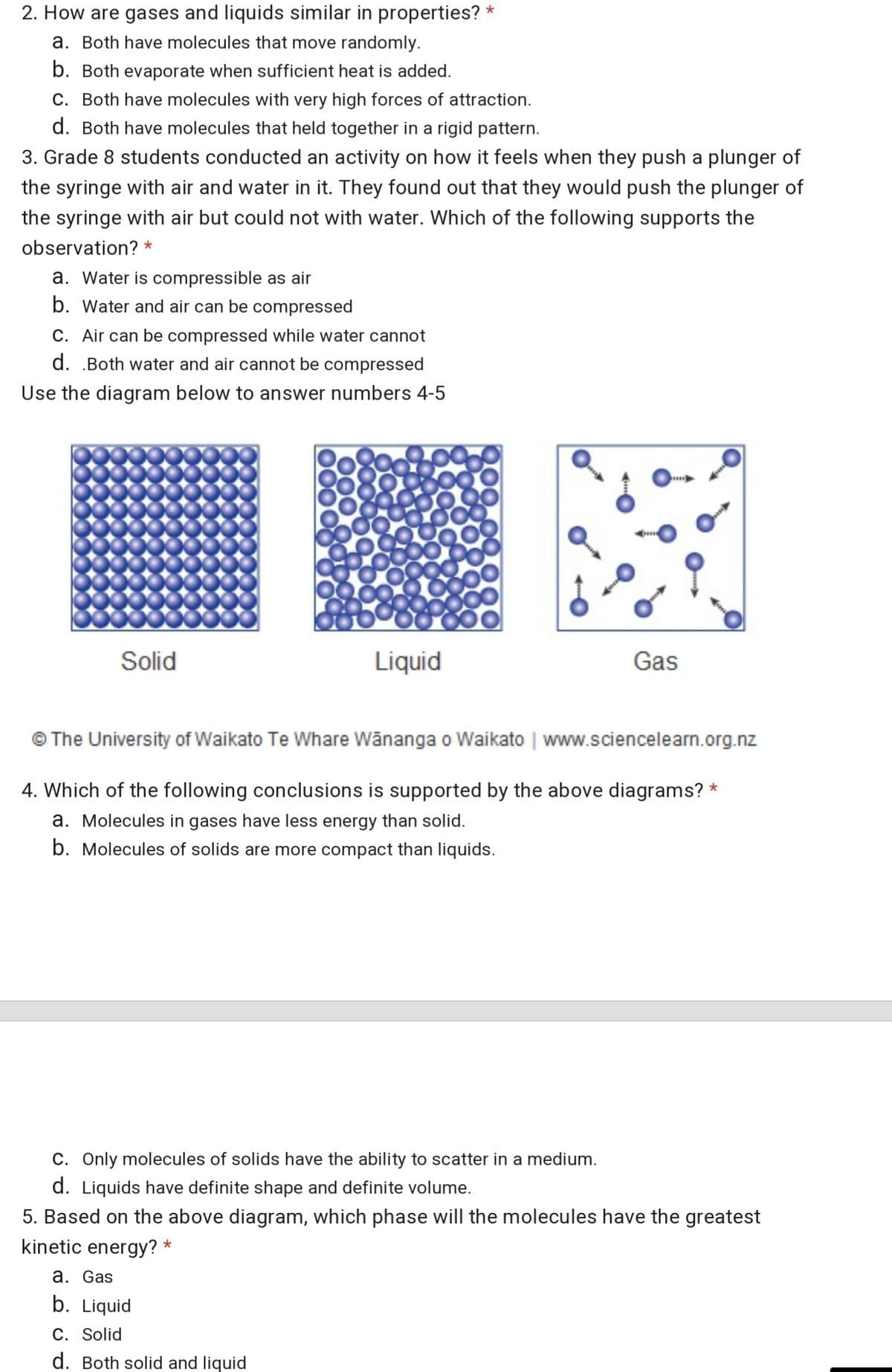
Transcribed Image Text:2. How are gases and liquids similar in properties?
a. Both have molecules that move randomly.
b. Both evaporate when sufficient heat is added.
C. Both have molecules with very high forces of attraction.
d. Both have molecules that held together in a rigid pattern.
3. Grade 8 students conducted an activity on how it feels when they push a plunger of
the syringe with air and water in it. They found out that they would push the plunger of
the syringe with air but could not with water. Which of the following supports the
observation? *
a. Water is compressible as air
b. Water and air can be compressed
C. Air can be compressed while water cannot
d. .Both water and air cannot be compressed
Use the diagram below to answer numbers 4-5
Solid
Liquid
Gas
© The University of Waikato Te Whare Wānanga o Waikato | www.sciencelearn.org.nz
4. Which of the following conclusions is supported by the above diagrams? *
a. Molecules in gases have less energy than solid.
b. Molecules of solids are more compact than liquids.
C. Only molecules of solids have the ability to scatter in a medium.
d. Liquids have definite shape and definite volume.
5. Based on the above diagram, which phase will the molecules have the greatest
kinetic energy? *
а. Gas
b. Liquid
C. Solid
d. Both solid and liquid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
