2. The industrial method currently used to produce butanol is the hydration of butene. C,Ha(g) + H,O(g) →> CH,„O(g) The enthalpy values for the following reactions are: 4C(s) + 4H,(g) → CHo(g) 4C(s) + 5H,(g) + ½0,(g) → C,H„O(g) 2H,(g) + O,(g) → 2H,0(g) AH = -7-1 kJ mo–1 AH = - 292-8 kJ mol–1 AΗ=-483-6 kl mol-1 Using the data above, calculate the enthalpy change, in kJ mol-1, to produce butanol by hydration of butene.
2. The industrial method currently used to produce butanol is the hydration of butene. C,Ha(g) + H,O(g) →> CH,„O(g) The enthalpy values for the following reactions are: 4C(s) + 4H,(g) → CHo(g) 4C(s) + 5H,(g) + ½0,(g) → C,H„O(g) 2H,(g) + O,(g) → 2H,0(g) AH = -7-1 kJ mo–1 AH = - 292-8 kJ mol–1 AΗ=-483-6 kl mol-1 Using the data above, calculate the enthalpy change, in kJ mol-1, to produce butanol by hydration of butene.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 36PS: Acetic acid. CH3CO2H, is made industrially by the reaction of methanol and carbon monoxide. CH3OH ()...
Related questions
Question
Hi, I hope you can help me with this. Thank you.
Instructions:
Show complete solution.
Observe organized calculation format. (Given, Required, Solution)
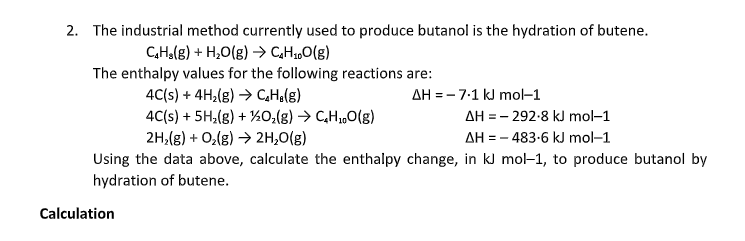
Transcribed Image Text:2. The industrial method currently used to produce butanol is the hydration of butene.
CHolg) + H,O(g) → CHz„O(g)
The enthalpy values for the following reactions are:
4C(s) + 4H,(g) → CHa(g)
4C(s) + 5H,(g) + ½0;(g) → C,H,„O(g)
2H,(g) + O,(g) → 2H,0(g)
AH = - 7:1 kJ mol-1
AH = - 292-8 kJ mol-1
AH = - 483-6 kJ mol-1
Using the data above, calculate the enthalpy change, in kJ mol-1, to produce butanol by
hydration of butene.
Calculation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning