2. Two analytical chemists have reported a method for monitoring the concentration of SO₂ in air. They compared their method to the standard method by analysing urban air samples collected from a single location. Samples were collected by drawing air through a collection solution for 6 min. Shown here is a summary of their results with SO₂ concentrations reported in mL/m³. standard method: new method: 21.62 22.20 24.27 23.54 24.25 23.09 21.02 21.54 20.51 24.62 25.72 21.54 22.31 21.30 Using an appropriate statistical test determine whether there is any significant difference between the standard method and the new method at a = 0.05.
2. Two analytical chemists have reported a method for monitoring the concentration of SO₂ in air. They compared their method to the standard method by analysing urban air samples collected from a single location. Samples were collected by drawing air through a collection solution for 6 min. Shown here is a summary of their results with SO₂ concentrations reported in mL/m³. standard method: new method: 21.62 22.20 24.27 23.54 24.25 23.09 21.02 21.54 20.51 24.62 25.72 21.54 22.31 21.30 Using an appropriate statistical test determine whether there is any significant difference between the standard method and the new method at a = 0.05.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 6CR: How is the pH scale defined? What range of pH values corresponds to acidic solutions? What range...
Related questions
Question
Please show all working.
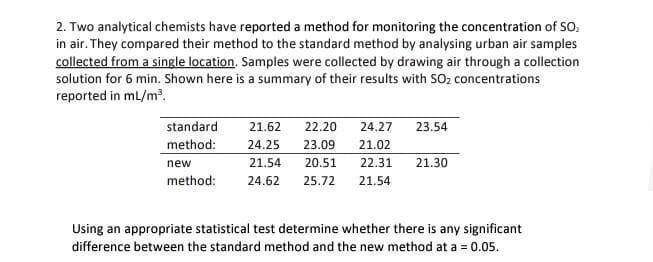
Transcribed Image Text:2. Two analytical chemists have reported a method for monitoring the concentration of SO₂
in air. They compared their method to the standard method by analysing urban air samples
collected from a single location. Samples were collected by drawing air through a collection
solution for 6 min. Shown here is a summary of their results with SO₂ concentrations
reported in mL/m³.
standard
method:
21.62 22.20 24.27
24.25 23.09 21.02
new
21.54 20.51
method: 24.62 25.72 21.54
23.54
22.31 21.30
Using an appropriate statistical test determine whether there is any significant
difference between the standard method and the new method at a = 0.05.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning