2. Use the Group I known mixture of Ag_Pb Hg.pdf to help identify which Group I metal cations are in your unknown. Your unknown code will be sent in a message via Canvas Oct 3. Open the Group I unknowns page and open the letter of your unknown from the message. Respond in the same text box as for questions 1,2, and 3 with Group I metal ions present in your unknown: metal ions absent: and How did you conclude these metal ions were present? (Write a sentence or two - do not be flippant or vague.)
2. Use the Group I known mixture of Ag_Pb Hg.pdf to help identify which Group I metal cations are in your unknown. Your unknown code will be sent in a message via Canvas Oct 3. Open the Group I unknowns page and open the letter of your unknown from the message. Respond in the same text box as for questions 1,2, and 3 with Group I metal ions present in your unknown: metal ions absent: and How did you conclude these metal ions were present? (Write a sentence or two - do not be flippant or vague.)
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 18RORR: The Ostwald process for the commercial production of nitric acid involves the following three steps:...
Related questions
Question

Transcribed Image Text:2. Use the Group I known mixture of Ag Pb Hg.pdf
_ to help identify which
Group I metal cations are in your unknown. Your unknown code will be sent in a
message via Canvas Oct 3. Open the Group I unknowns page and open the letter
of your unknown from the message.
Respond in the same text box as for questions 1,2, and 3 with
Group I metal ions present in your unknown:
metal ions
absent:
and
How did you conclude these metal ions were present? (Write a sentence or
two - do not be flippant or vague.)
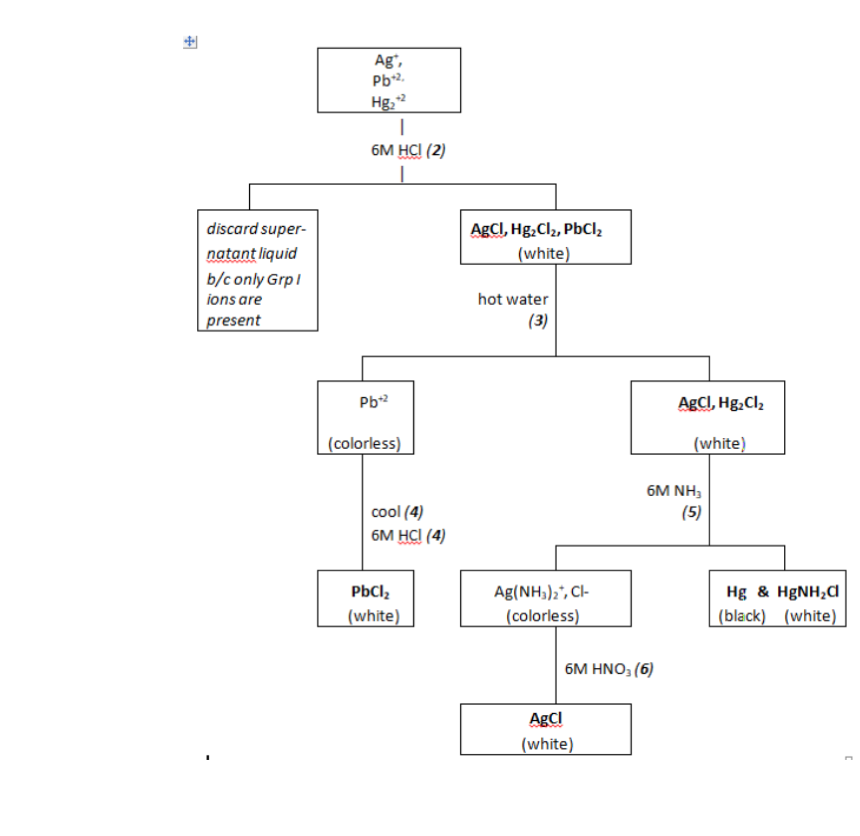
Transcribed Image Text:Ag",
Pb2.
Hg,
6м Hа (2)
discard super-
ABCI, Hg,Cl2, PbCI,
natant liquid
(white)
b/c only GrpI
ions are
hot water
present
(3)
Pb2
AgCI, Hg. Cl2
(colorless)
(white)
6M NH,
cool (4)
6M HC! (4)
(5)
Ag(NH,)2", Cl-
(colorless)
PbCl,
Hg & HØNH,d
(white)
(black) (white)
6M ΗΝΟ, (6)
AgCI
(white)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning