(a) FeySa(s) and HBr(aq) Express your answer as a balanced net ionic equation. Identify all of the phases in your answer. ΑΣφ DA chemical reaction does not occur for this question. Submit Request Answer Part B K2CO3 (aq) and CuCl2 (ag) Express your answer as a balanced net ionic equation. Identify all of the phases in your answek Write "N.R." if no reaction occurs. ΑΣφ ? DA chemical reaction does not occur for this question. Submit Request Answer
(a) FeySa(s) and HBr(aq) Express your answer as a balanced net ionic equation. Identify all of the phases in your answer. ΑΣφ DA chemical reaction does not occur for this question. Submit Request Answer Part B K2CO3 (aq) and CuCl2 (ag) Express your answer as a balanced net ionic equation. Identify all of the phases in your answek Write "N.R." if no reaction occurs. ΑΣφ ? DA chemical reaction does not occur for this question. Submit Request Answer
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter2: Matter
Section: Chapter Questions
Problem 23A
Related questions
Question
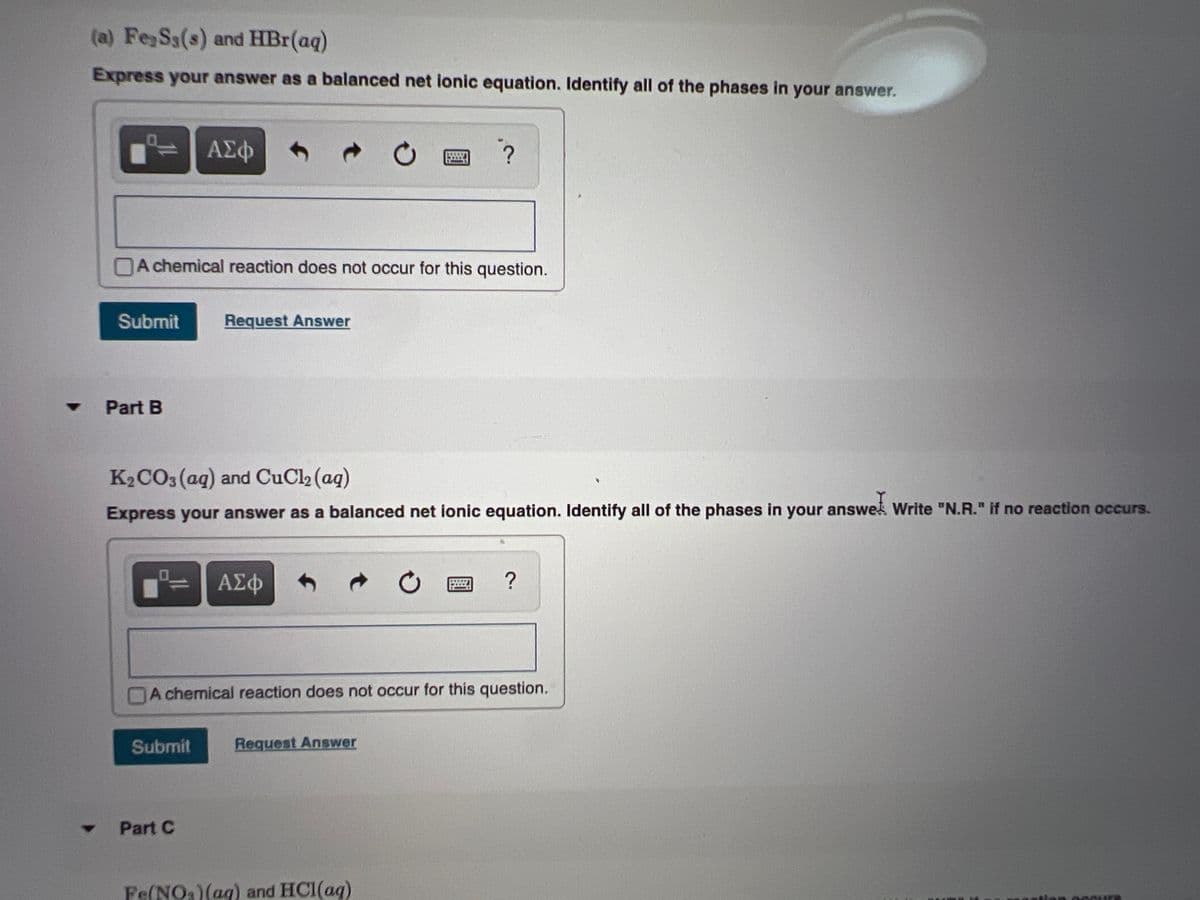
Transcribed Image Text:(a) Fe Ss(s) and HBr(aq)
Express your answer as a balanced net ionic equation. Identify all of the phases in your answer.
ΑΣφ
A chemical reaction does not occur for this question.
Submit
Request Answer
Part B
K2 CO3(aq) and CuCl2 (aq)
Express your answer as a balanced net ionic equation. Identify all of the phases in your answel Write "N.R." if no reaction occurs.
ΑΣφ
?
A chemical reaction does not occur for this question.
Submit
Request Answer
Part C
Fe(NO)(ag) and HCI(ag)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole