2. What were your second assigned reactants in Part A? Sobium Phosphatel Zine Nitrate Reactant A Reactant B a. Write the balanced complete themical equation, the complete ionic equation, and net ionic equation for this reaction.
2. What were your second assigned reactants in Part A? Sobium Phosphatel Zine Nitrate Reactant A Reactant B a. Write the balanced complete themical equation, the complete ionic equation, and net ionic equation for this reaction.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 40P
Related questions
Question
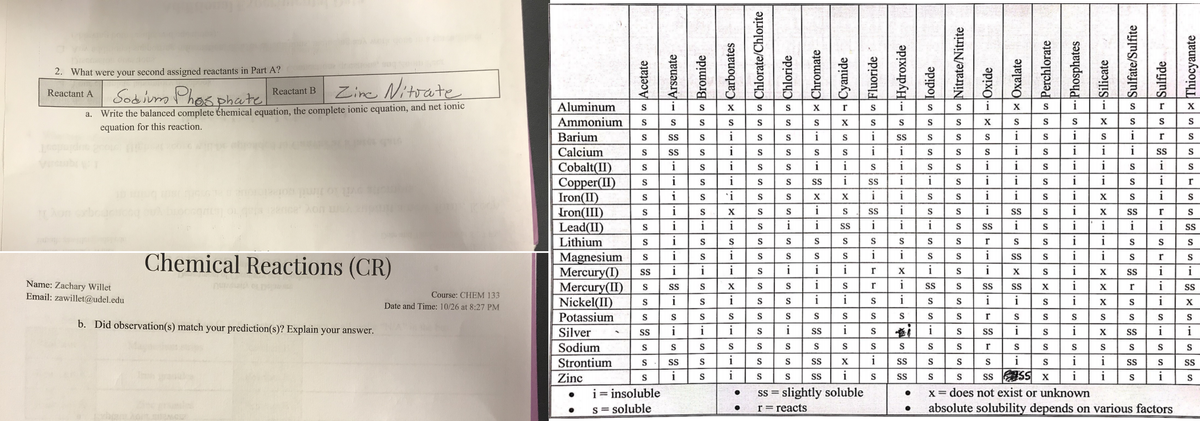
Transcribed Image Text:2. What were your second assigned reactants in Part A?
Sobium Phosphate
Zine Nitoate
Reactant A
Reactant B
i
i
i
Write the balanced complete themical equation, the complete ionic equation, and net ionic
equation for this reaction.
Aluminum
S
i
X
S
S
X
S
S
X
S
X
а.
Ammonium
S
S
S
S
S
S
S
S
S
S
S
S
S
S
S
Barium
S
S
S
i
S
S
i
S
SS
S
S
S
i
S
i
S
S
i
i
i
i
i
Calcium
Cobalt(II)
Copper(II)
Iron(II)
Iron(III)
Lead(II)
Lithium
S
S
S
S
S
S
S
S
S
SS
S
i
i
i
i
i
i
i
i
i
i
S
S
S
S
S
S
i
i
i
S
i
S
i
S
SS
i
SS
i
i
i
S
i
i
i
S
i
S
i
S
S
X
i
i
S
S
i
S
i
i
S
S
i
S
X
S
i
S
S
i
S
S
SS
i
S
S
i
i
i
i
i
SS
i
i
i
SS
i
S
i
i
i
SS
S
i
S
S
S
S
S
S
S
S
S
S
S
S
i
i
S
i
i
i
i
i
Chemical Reactions (CR)
i
i
Magnesium
Mercury(I)
Mercury(II)
Nickel(II)
Potassium
S
S
S
S
S
SS
S
S
S
i
i
i
i
i
i
i
i
i
i
X
S
S
X
S
i
i
i
Name: Zachary Willet
Email: zawillet@udel.edu
S
SS
X
S
r
SS
SS
SS
X
r
i
SS
Course: CHEM 133
S
i
i
S
S
i
S
S
S
i
i
i
X
S
i
Date and Time: 10/26 at 8:27 PM
S
S
S
S
S
S
S
S
S
S
S
S
S
S
S
S
b. Did observation(s) match your prediction(s)? Explain your answer.
Silver
S
i
i
i
i
SS
i
S
i
S
SS
i
S
i
X
S
i
i
Sodium
S
S
S
S
S
S
S
S
S
S
S
Strontium
i
i
i
i
i
S
SS
S
S
S
SS
SS
S
S
S
S
SS
ss SS x
does not exist or unknown
absolute solubility depends on various factors
Zinc
S
i
S
i
S
S
S
i
S
SS
S
i
i
i
S
i= insoluble
Ss = slightly soluble
X=
s = soluble
r=reacts
A--s Fluoride
---|--- Phosphates
n-A |s n---|s Sulfate/Sulfite
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning