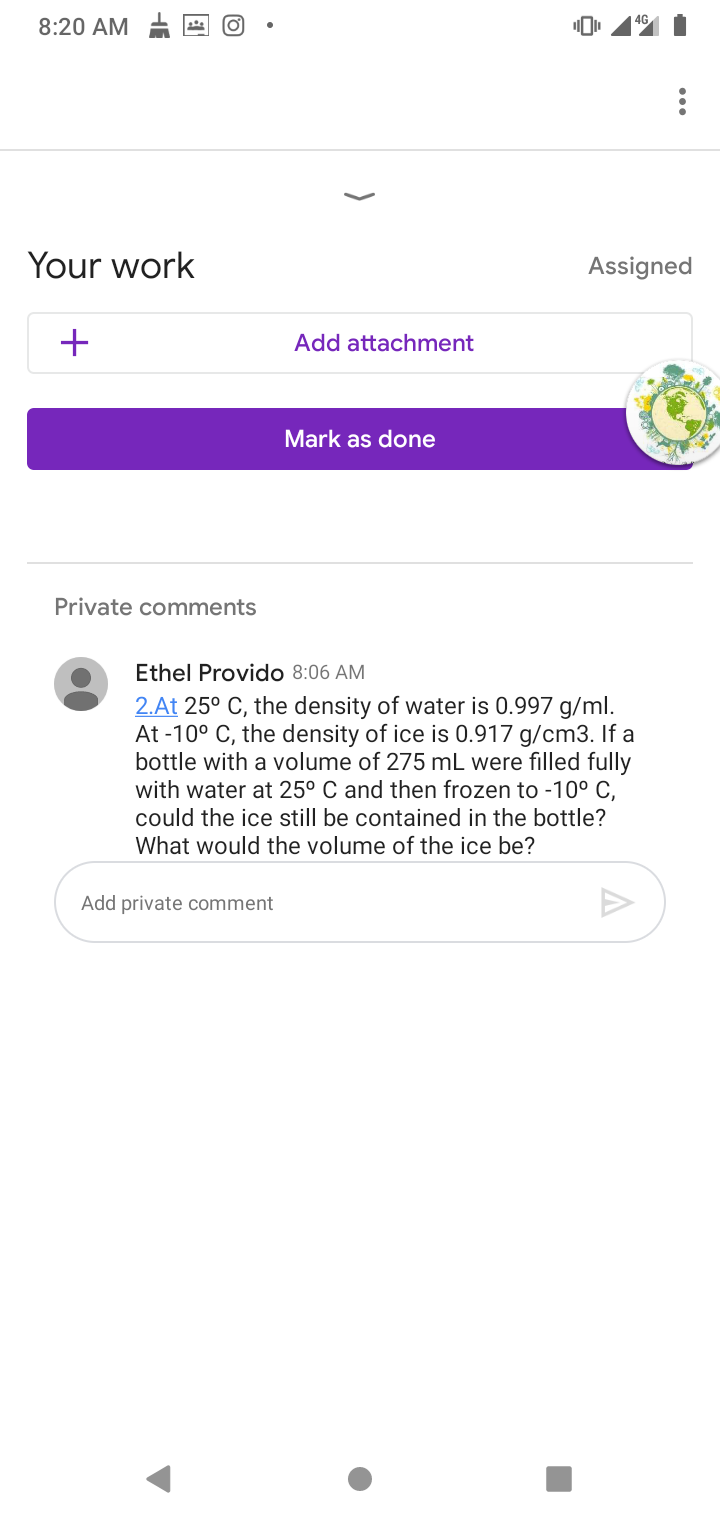
2.At 25° C, the density of water is 0.997 g/ml. At -10° C, the density of ice is 0.917 g/cm3. If a bottle with a volume of 275 mL were filled fully with water at 25° C and then frozen to -10° C, could the ice still be contained in the bottle? What would the volume of the ice be?
2.At 25° C, the density of water is 0.997 g/ml. At -10° C, the density of ice is 0.917 g/cm3. If a bottle with a volume of 275 mL were filled fully with water at 25° C and then frozen to -10° C, could the ice still be contained in the bottle? What would the volume of the ice be?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 43GQ: Hexane (C6H14, density = 0.766 g/cm3), perfluoro-hexane (C6F14, density = 1.669 g/cm3), and water...
Related questions
Question
100%
Transcribed Image Text:4G
8:20 AM
Your work
Assigned
+
Add attachment
Mark as done
Private comments
Ethel Provido 8:06 AM
2.At 25° C, the density of water is 0.997 g/ml.
At -10° C, the density of ice is 0.917 g/cm3. If a
bottle with a volume of 275 mL were filled fully
with water at 25° C and then frozen to -10° C,
could the ice still be contained in the bottle?
What would the volume of the ice be?
Add private comment
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning