20. A chemist decomposes several samples of water into hydro- gen and oxygen and measures the mass of the hydrogen and the oxygen obtained. The results are tabulated as follows. Sample Number Grams of Hydrogen Grams of Oxygen 1 1.5 12 2 2 16 2.5 20 (a) Summarize these observations in a short statement. Next, the chemist decomposes several samples of carbon dioxide into carbon and oxygen. The results are tabulated as follows: Sample Number Grams of Carbon Grams of Oxygen 1 0.5 1.3 2 1.0 2.7 1.5 4.0 (b) Summarize these observations in a short statement. (c) Formulate a law from the observations in (a) and (b). (d) Formulate a theory that might explain your law in (c).
20. A chemist decomposes several samples of water into hydro- gen and oxygen and measures the mass of the hydrogen and the oxygen obtained. The results are tabulated as follows. Sample Number Grams of Hydrogen Grams of Oxygen 1 1.5 12 2 2 16 2.5 20 (a) Summarize these observations in a short statement. Next, the chemist decomposes several samples of carbon dioxide into carbon and oxygen. The results are tabulated as follows: Sample Number Grams of Carbon Grams of Oxygen 1 0.5 1.3 2 1.0 2.7 1.5 4.0 (b) Summarize these observations in a short statement. (c) Formulate a law from the observations in (a) and (b). (d) Formulate a theory that might explain your law in (c).
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter2: Matter
Section: Chapter Questions
Problem 10A
Related questions
Question
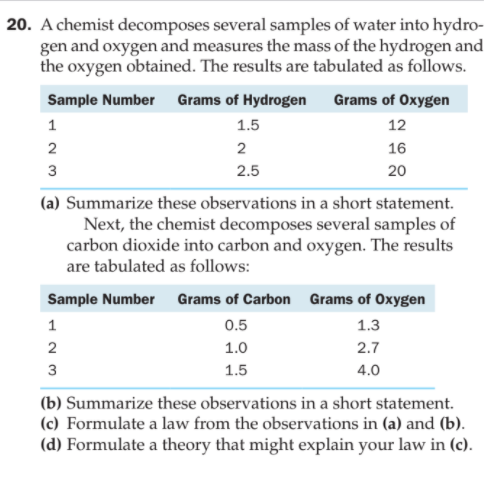
Transcribed Image Text:20. A chemist decomposes several samples of water into hydro-
gen and oxygen and measures the mass of the hydrogen and
the oxygen obtained. The results are tabulated as follows.
Sample Number
Grams of Hydrogen
Grams of Oxygen
1
1.5
12
2
2
16
2.5
20
(a) Summarize these observations in a short statement.
Next, the chemist decomposes several samples of
carbon dioxide into carbon and oxygen. The results
are tabulated as follows:
Sample Number
Grams of Carbon Grams of Oxygen
1
0.5
1.3
2
1.0
2.7
1.5
4.0
(b) Summarize these observations in a short statement.
(c) Formulate a law from the observations in (a) and (b).
(d) Formulate a theory that might explain your law in (c).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning
