20. A diagram representing water molecules in the solid phase (ice) is shown below. Which of these diagrams best shows what water would look like after it melts (changes to a liquid)? * A. B а. А b. в С. С d. D 21. Which of the following involves a change of state in matter? * a. Adding food color to water b. From clouds to raindrops C. Placing a candy bar on scale d. Chopping of wood 22. The process by which the molecules of a solid turns immediately to gas without passing the liquid state is known as a. Condensation b. Freezing C. Melting d. Sublimation 23. A glass full of water was left on top of a table. After several days, the amount of water in the glass decreased. What process took place with the water in the glass? * a. Condensation b. Evaporation C. Solidification d. Sublimation 24. A liquid boils at 100°C. Which other property of a liquid proves that it is pure water? * a. It freezes at 0°C. b. It is neither acidic nor basic. C. It does not leaves a residue when boiled. d. It turns blue dish washing liquid to red.
20. A diagram representing water molecules in the solid phase (ice) is shown below. Which of these diagrams best shows what water would look like after it melts (changes to a liquid)? * A. B а. А b. в С. С d. D 21. Which of the following involves a change of state in matter? * a. Adding food color to water b. From clouds to raindrops C. Placing a candy bar on scale d. Chopping of wood 22. The process by which the molecules of a solid turns immediately to gas without passing the liquid state is known as a. Condensation b. Freezing C. Melting d. Sublimation 23. A glass full of water was left on top of a table. After several days, the amount of water in the glass decreased. What process took place with the water in the glass? * a. Condensation b. Evaporation C. Solidification d. Sublimation 24. A liquid boils at 100°C. Which other property of a liquid proves that it is pure water? * a. It freezes at 0°C. b. It is neither acidic nor basic. C. It does not leaves a residue when boiled. d. It turns blue dish washing liquid to red.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 14CR
Related questions
Question
Answer number 20 to 24 pls. I don't want to waste my money here.
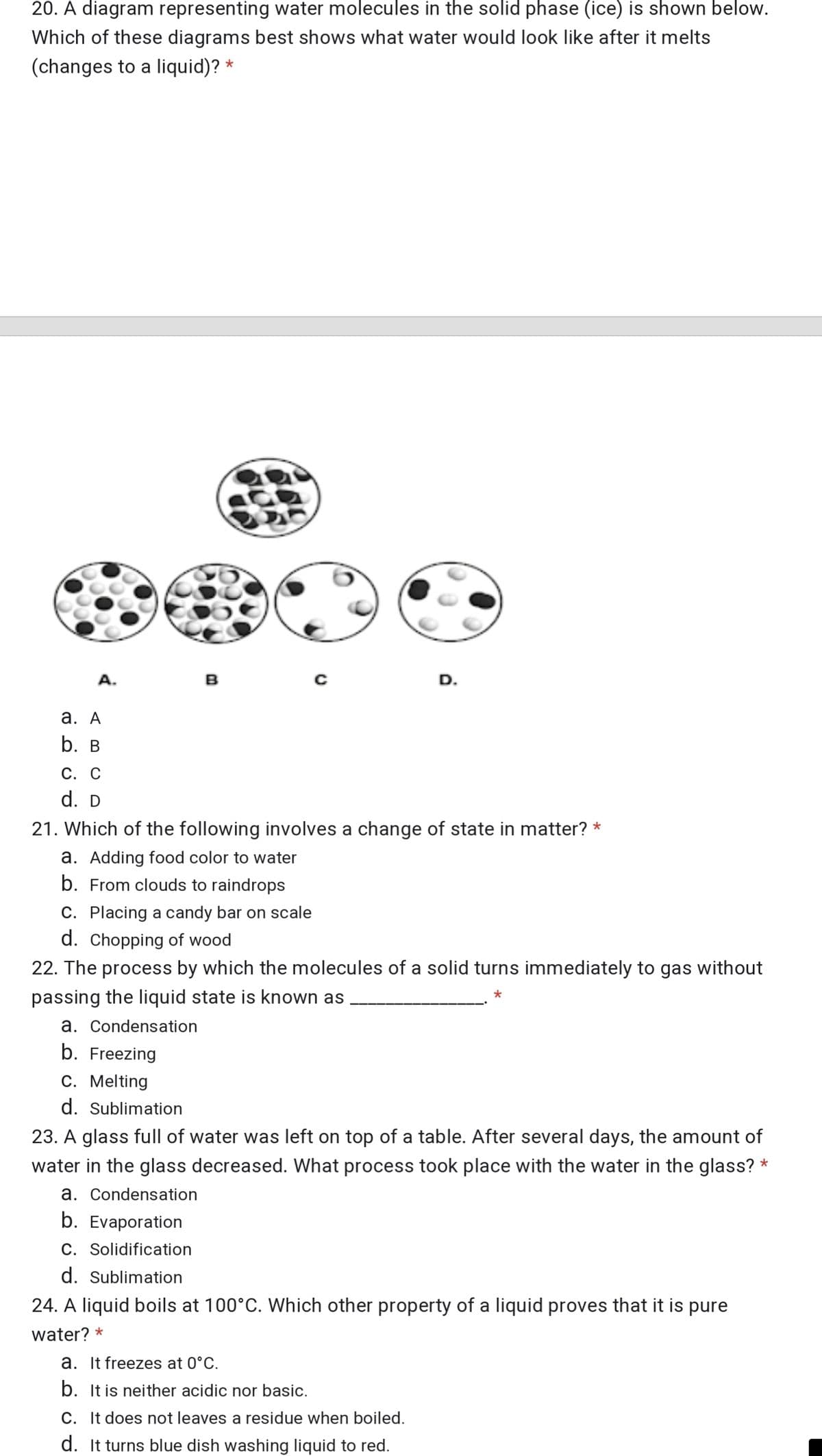
Transcribed Image Text:20. A diagram representing water molecules in the solid phase (ice) is shown below.
Which of these diagrams best shows what water would look like after it melts
(changes to a liquid)? *
A.
в
D.
а. А
b. B
С. С
d. D
21. Which of the following involves a change of state in matter? *
a. Adding food color to water
b. From clouds to raindrops
C. Placing a candy bar on scale
d. Chopping of wood
22. The process by which the molecules of a solid turns immediately to gas without
passing the liquid state is known as
a. Condensation
b. Freezing
C. Melting
d. Sublimation
23. A glass full of water was left on top of a table. After several days, the amount of
water in the glass decreased. What process took place with the water in the glass? *
a. Condensation
b. Evaporation
C. Solidification
d. Sublimation
24. A liquid boils at 100°C. Which other property of a liquid proves that it is pure
water? *
a. It freezes at 0°C.
b. It is neither acidic nor basic.
C. It does not leaves a residue when boiled.
d. It turns blue dish washing liquid to red.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER