200g zinc bromide reacts completely with excess magnesium nitrate to producing zinc nitrate and magnesium bromide how much of each product will be formed
200g zinc bromide reacts completely with excess magnesium nitrate to producing zinc nitrate and magnesium bromide how much of each product will be formed
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 6ALQ: You have a chemical in a sealed glass container filled with air. The setup is sitting on a balance...
Related questions
Question
200g zinc bromide reacts completely with excess magnesium nitrate to producing zinc nitrate and magnesium bromide how much of each product will be formed?
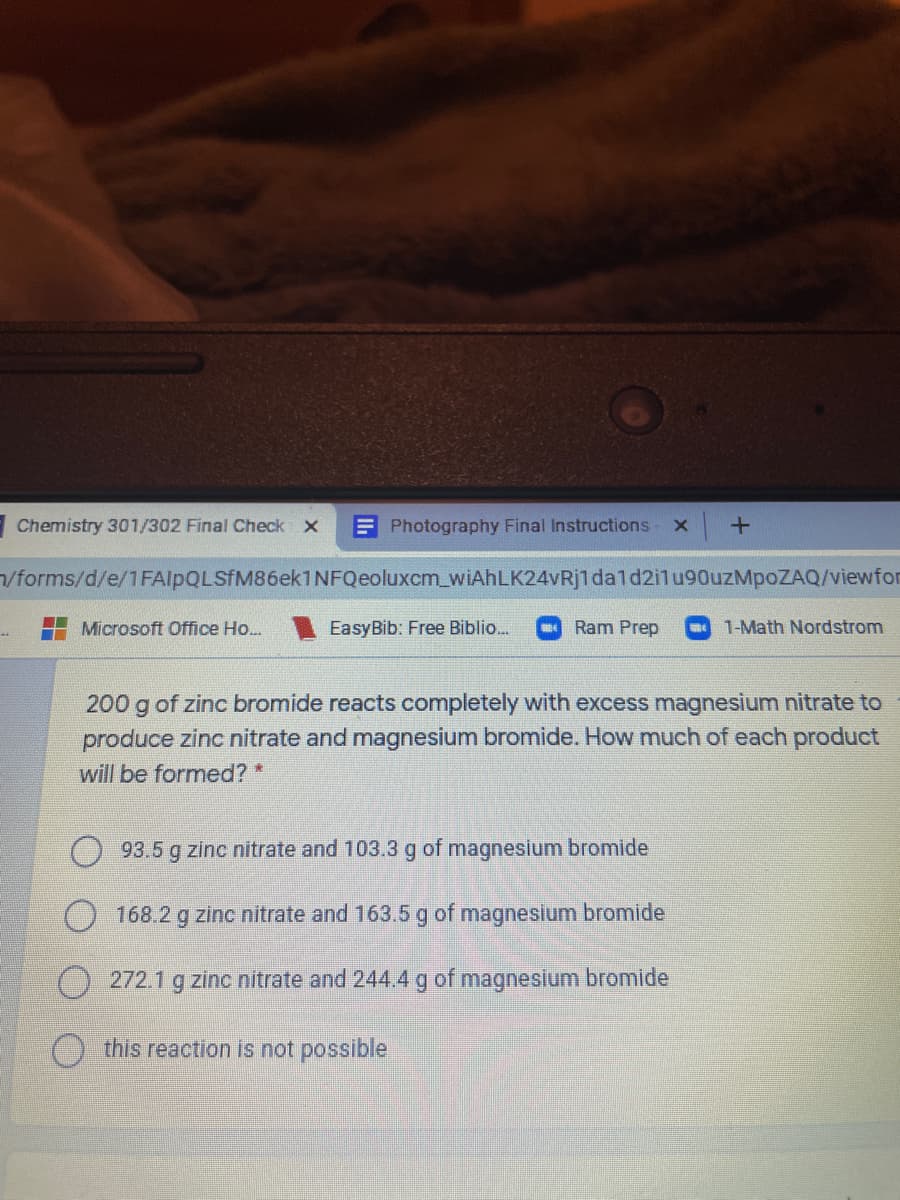
Transcribed Image Text:Chemistry 301/302 Final Check X
E Photography Final Instructions
/forms/d/e/1FAlpQLSfM86ek1NFQeoluxcm_wiAhLK24vRj1dald2ilu90uzMpoZAQ/viewfor
Microsoft Office Ho...
EasyBib: Free Biblio.
Ram Prep
1-Math Nordstrom
200 g of zinc bromide reacts completely with excess magnesium nitrate to
produce zinc nitrate and magnesium bromide. How much of each product
will be formed? *
93.5 g zinc nitrate and 103.3 g of magnesium bromide
) 168.2 g zinc nitrate and 163.5 g of magnesium bromide
272.1 g zinc nitrate and 244.4 g of magnesium bromide
this reaction is not possible
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning