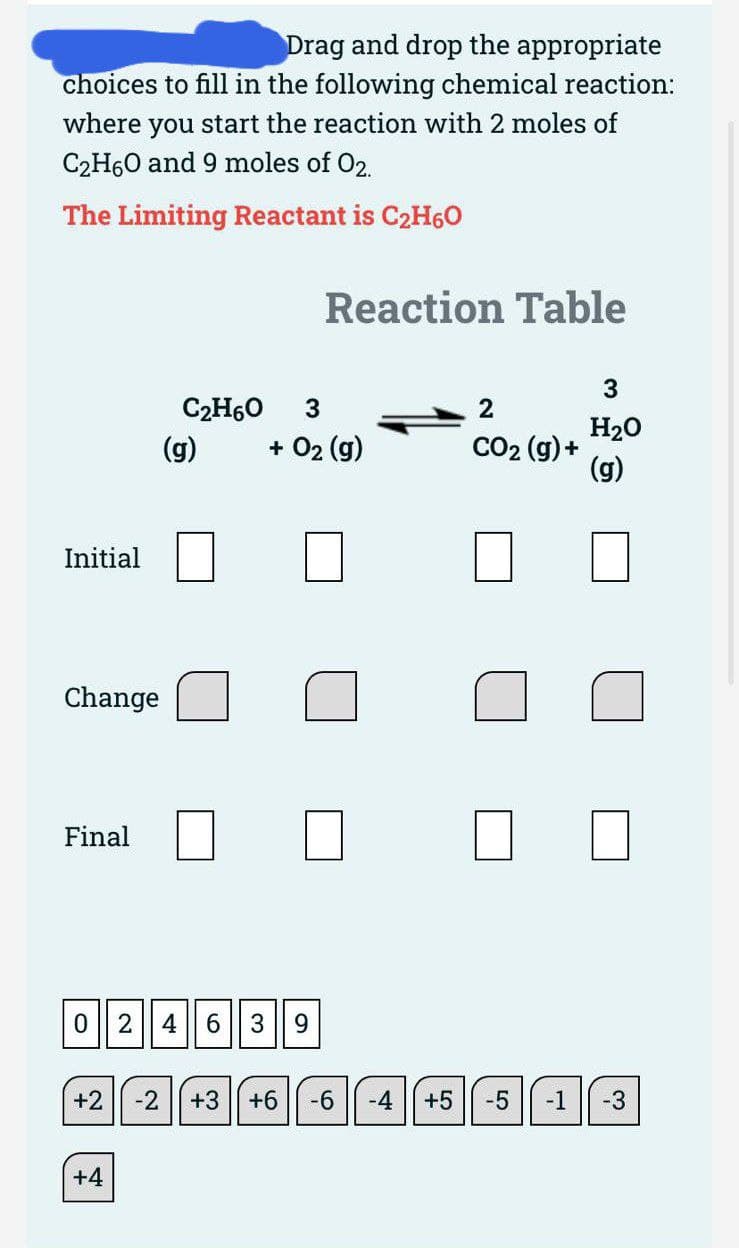
where you start the reaction with 2 moles of C2H60 and 9 moles of 02. The Limiting Reactant is C2H60 Reaction Table C2H60 3 2 H20 CO2 (g) + (g) (g) + 02 (g) Initial
Q: For each of the species below, identify any cyclic conjugated system, then: A. Determine the number…
A: For aromatic anti-aromatic cyclic and planar structure is important condition....
Q: Diffusion of Gases Consider that you carried out the following, where you 1. Place 1.0 mL of dilute…
A: Phenolphthalein is an indicator that is used for the detection of acidic or basic medium. The pH…
Q: ne concentration in um
A:
Q: Provide the correct sequence of reactions needed form the epoxide starting from ethyne. from H, H EH…
A: ->NaNH2 is base which can abstract most acidic hydrogen present in terminal Alkyne and form…
Q: esti mist titrates 20.0 ml of 0.20 M HBIO with 0.10 M NHOH. Galculate the pH: 1. Before the…
A: a.) Before titration there is only acid , so we would use dissociation equation of acid to calculate…
Q: bn the lub vou titrated 40 ml of 0.006 M Ca with 0.008 M EDTA at pH 10. What was the pCa in that…
A:
Q: for this question. A sample of nitrogen gas at a pressure of 1.03 atm and a temperature of 22.4 °C,…
A:
Q: A sample of an unknown compound is vaporized at 120. °C. The gas produced has a volume of 1400. mL…
A:
Q: Be sure to answer all parts. Complete the following nuclear equations and identify X in each case:…
A:
Q: A Haber process reactor contains 25.0% nitrogen and 75.0% hydrogen for the synthesis of ammonia.…
A:
Q: The molar solubility of iron(II) carbonate in a 0.110 M iron(II) nitrate solution is М.
A: Given :: Molarity of Fe(NO3)2 = 0.110 M Ksp of FeCO3 =3.07×10-11 Molar solubility (S) of FeCO3 in…
Q: What is the solubility (in g/L) of lead(II) chromate, PbCrO4 in (2.600x10^-1)M potassium chromate?…
A: Given: The Ksp for lead(II) chromate = 1.8x10-14\ We have to calculate the solubility (in g/L) of…
Q: Q11. Provide a mechanism that leads to the major product: NO2 CH NH2 Br Q12. Propose a set of…
A: Q 11. Aromatic Sn2. Q 12. ( see below).
Q: from H =H + enatiomer Br 3. Hz, Pd, Lindlar's catalyst 4. H,C A 1. NANH2, NH, 2. Br 1. NANH2, NH, 2.…
A: ->NaNH2 is base which can abstract most acidic hydrogen and form nucleophile.which can give…
Q: Consider the apparatus shown below with the valve closed. The volume and pressure of each gas is…
A:
Q: A voltaic cell is 'constructed from a standard AlT|Al half cell (E° red -1.660V) and a standard Mn+…
A: Given that, a voltaic cell is constructed from a standard Al3+|Al half-cell (E0red = -1.660 V) and a…
Q: Draw the structure(s) of the major organic product(s) of the following reaction. Dilute aqueous HCI
A:
Q: Mixtures are divided into two classes based O 1. mixtures and matter on their appearance. What are…
A:
Q: Molecule SO2 Lewis Structure 3D representation Bond Angles Polarity (if yes, show dipole) 6+2(6) ö=s…
A:
Q: How does water activity affect enzymatic reaction?
A: The action of enzymes to carry out a reaction is known as enzymatic action. It depends on the…
Q: Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone…
A: The aldehyde or ketone having alpha hydrogen undergo condensation reaction in presence of base like…
Q: 3. A 50.0 mL sample of 0.25 M HCI was mixed with 100.0 mL sample of 0.40 M sodium hypochlorite. What…
A: Given :- Volume of sodium hypochlorite, NaClO = 100.0 mL = 0.100 L Molarity of sodium…
Q: aromatic, antiaromatic, or non aromatic molecule?
A:
Q: A sample of an unknown gas with a molar mass of 23.31 is placed in a vessel with a volume of 1,792…
A: Weight of gas is calculated by using ideal gas equation
Q: Draw and label a structure of: A diglyceride of oleic acid
A: Diglyceride of oleic acid have the structural formula
Q: How might the following compound be prepared using a Michael reaction? Draw both the Michael donor…
A:
Q: Will the following reaction be spontaneous under standard conditions? Zn(s) + 2H*(aq) → Zn+2(aq) +…
A:
Q: prational temper aracteristic rota lculate the parti tational and vibr andard molar er
A: Given that characteristic vibration temperature is= 3084K Thus ,vibration partition function of CO…
Q: Assign oxidation numbers to each element and balance the equation in an acidic solution. Cr2O72- +…
A: The balanced equation in acidic medium is given below
Q: (a) EtO OEt NH2 (c) OEt
A: Detail synthetic route is given below
Q: 72 The process of wine making involves the fermentation of glucose, where 650 mL of C0, gas was…
A: Given: The volume of CO2 gas produced at 37 ∘C and 1.00 atm = 650 mL We have to find the volume of…
Q: 0.00 L tank at 5.2 °C is filled with 12.9 g of boron trifluoride gas and 2.08 g of dinitrogen…
A: Given-> Volume = 10.00 L T = 5.2°C = 5.2 +273 = 278.2 K Weight of BF3 = 12.9 gm Weight of N2F2 =…
Q: Consider the reaction: X2(g)→2X(g). When a vessel initially containing 763 torr of X2 comes to…
A:
Q: Briefly describe how a phase-contrast microscope work and the kind of image that it produces. Give a…
A: Phase-contrast microscopy generates an image by using the different refractive indexes and densities…
Q: For which of these pairs of species is the first species an acid AND the second species is its…
A:
Q: : It is desired to use a conductance apparatus to measure the concentration of dilute solution of…
A:
Q: Assign oxidation numbers to each element and balance the equation in a basic solution. H2O2 + ClO2…
A: The balanced equation in basic medium is given below
Q: Give IUPAC names for the following compounds: SCH(CH3)2
A: The naming of an organic compound can be done with help of rules of international union of pure and…
Q: QUESTION 6 If 47.91 mL of distilled water at 99.90 °C is added to 49.00 mL of distilled water, in a…
A:
Q: The graph below shows the distribution of molecular speeds in a sample of fluorine gas at…
A: The distribution of the speed of gas molecules at various temperatures can be understood by the…
Q: Find calculated k value per trial: Average k value: The initial volume of syringe was 15ml and I…
A: To find calculated average k value per trial and average k value.
Q: 729 Identify the relationship in each of the following pairs. Do the drawings represent consti-…
A:
Q: Choose the right reagent or series of reagents from the ones listed below to prepare 2-pentanone…
A: Detail mechanistic pathway is given below
Q: What hazards should you be aware of when working with copper and lead compounds in the laboratory
A: High levels of copper and lead compounds can be harmful. Inhaling large amounts of copper or lead…
Q: The resistance of a saturated solution of PBSO4 (very insoluble salt) at 291K is of 1.7x106 0, being…
A: The resistance defines the hindrance to the mobility of the ions in a solution and it can be…
Q: Draw the Lewis structure of KrF, and then determine the hybridization of the central atom. A) sp B)…
A: Lewis’s structure: Lewis’s bonding theory is based on the octet rule. The Lewis structure is a…
Q: How many grams of silver can be plated onto an object in 5.70 minutes at 7.17 A of current? Ag*(aq)…
A: The amount of Silver plated is given below
Q: 10. Alkyl diazonium salts decompose to form carbocations, which go on to form products of…
A:
Q: Consider these two entries from a fictional table of standard reduction potentials.…
A: We have to calculate the standard potential of cell.
Q: What is the pH of a solution made by mixing 100.mL of 0.0500 M HCIO4lan) and 50.0mL 0.0400 M KOH(an?…
A:
Step by step
Solved in 2 steps with 1 images

- NH4+ {aq) + NO2(aq) -> N2(g) +2H2O{l} Data Initial [NH4+] Initial [NO2-] rate 1 0.0100 0.200 5.4 x10-7 2 0.0200 0.200 10.8x10-7 3 0.0400 0.200 21.5x10-7 4 0.200 0.0202 10.8x10-7 5 0.200 0.0404 21.6x10-7 6 0.200 0.0808 43.3x10-7 Find x,y,kWhat is the theoretical yield of ethyl chloride in the reaction of 20.2 gg of ethylene with 48 gg of hydrogen chloride? (For ethylene, MW=28.0amuMW=28.0amu; for hydrogen chloride, MW=36.5amuMW=36.5amu; for ethyl chloride, MW=64.5amuMW=64.5amu.)H2C=CH2+HCl→CH3CH2ClH2C=CH2+HCl→CH3CH2ClA student obtained 2.111 g of a mixture containing four components. After performing separation by chemical and physical methods, the student obtained the following mass data: Component 1: 0.486 g Component 2: 0.317 g Component 3: 0.127 g Component 4: 0.929 g Calculate the total percent recovery of the mixture. (Please show work)
- 1) Commercial fuming Sulphuric acid (Oleum-H2S2O6) is 99.9% solution. Please convert it into molarity.2) Find out the Volume (dm3) of product (gas) at RTP when 0.58 M, 150 mL NaOH (aq.) reacts with 350 mL, 0.25 NH4Cl.WATCH THIS TWO MINUTE VIDEO TO SOLVE https://www.youtube.com/watch?v=9Wxxv7kwXlA&list=PLE-217H0ao60Uzpzj-FNP_0ScVp5SZB7j&index=8 What is the theoretical yield of this experiment? 1.217g 49% 4.9g .0893gEugenol, the main component of clove oil, can be prepared by heating 2-allyl guaiacol (in the absence of oxygen) to temperatures >205 °C. In practice, however, it is most cost-effective to extract eugenol from cloves, its natural source. Given a weight percentage of eugenol in cloves at 15.0%, calculate the minimum mass of cloves one needs to obtain 8.00 g of eugenol as a product. Report your answer (as a mass in grams, without units) with three significant digits.
- Extraction of Caffeine Post-lab 5. On the report sheet, you calculated your percent recovery of caffeine. a. If your percent was less than 90%, give at least one reason why it was less. b. A group of students have their percent yield more than 100%, give at least one reasonwhy it was more than 100%. Do NOT simply say “human error.” Be specific about the typesof errors (human or otherwise) that may have occurred, leading to a recovery more than eorequal to 100% (Is this possible Why?) 6. Based on what you observed during this experiment, is dichloromethane denser OR lessdense than water? Explain your reasoning. 7. Based on what happened in this experiment, is caffeine more soluble in water OR moresoluble in dichloromethane? Explain your reasoning.Airbag experiment The simulation link is provided below if needed. It's probably best to use the simulation because the pictures attached won't show everything in full. Simulation link: https://interactives.ck12.org/simulations/chemistry/decomposition-reaction/app/index.html?screen=sandbox&lang=en&referrer=ck12Launcher&backUrl=https://interactives.ck12.org/simulations/chemistry.html Just select ammonium nitrate and then select 1 mole and press play. Record macroscopic observations of the bag and the crash test dummy as well as microscopic observations of the particles within the steering mechanism and the inflated/inflating airbag.What is the simplest CGS unit for "poise"?
- Airbag experiment The simulation link is provided below if needed. It's probably best to use the simulation because the pictures attached won't show everything in full. Simulation link: https://interactives.ck12.org/simulations/chemistry/decomposition-reaction/app/index.html?screen=sandbox&lang=en&referrer=ck12Launcher&backUrl=https://interactives.ck12.org/simulations/chemistry.html Just select ammonium nitrate and then select 2 moles and press play. Record macroscopic observations of the bag and the crash test dummy as well as microscopic observations of the particles within the steering mechanism and the inflated/inflating airbag.1. Find HCl Mol weight 2. Find NH3 Mol. weight 3. HCI Diff. Distance: 10 4. NH3Diff. Distance: 60 5. Find rate ration (exp.) : Rate ration =Distance A/Distance B 6. Find rate ration (theor.) = MWb/MWaHow would each of the following errors affect the determination of the molar mass of the unknown (Increase/Decrease/No effect)? a. Thermometer reads 2.0o higher than the true temperature. b. Some of the t-butanol was unknowingly spilled after it had been weighed but before the solute was added. 3. A student accidentally added acetylsalicylic acid (MW = 180.157 g/mol) rather than salicylic acid (MW = 138.121 g/mol), if no other mistakes were made, how would this error affect the determined molal freezing point depression constant for t-butyl alcohol (Kf)? 4. Based on the Tf and Kf you determined for t-butyl alcohol, predict the freezing point of a t-butyl alcohol solution containing 0.530 m NaCl. Assume 1.9 is the van’t Hoff factor for NaCl in t-butyl alcohol. Show your work.

