Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter10: Atomic Emission Spectrometry
Section: Chapter Questions
Problem 10.11QAP
Related questions
Question
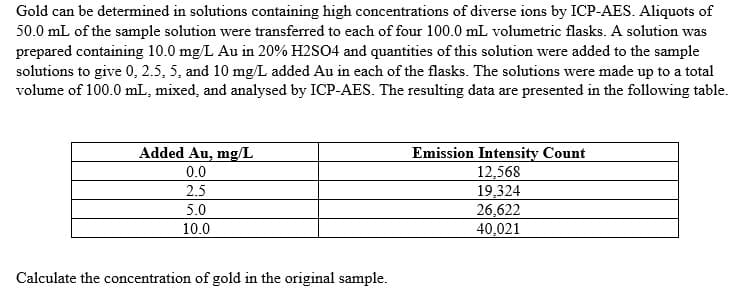
Transcribed Image Text:Gold can be determined in solutions containing high concentrations of diverse ions by ICP-AES. Aliquots of
50.0 mL of the sample solution were transferred to each of four 100.0 mL volumetric flasks. A solution was
prepared containing 10.0 mg/L Au in 20% H2SO4 and quantities of this solution were added to the sample
solutions to give 0, 2.5, 5, and 10 mg/L added Au in each of the flasks. The solutions were made up to a total
volume of 100.0 mL, mixed, and analysed by ICP-AES. The resulting data are presented in the following table.
Added Au, mg/L
Emission Intensity Count
12,568
19,324
26,622
40,021
0.0
2.5
5.0
10.0
Calculate the concentration of gold in the original sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning