
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 14, Problem 14.2QAP
A 0.4740-g pesticide sample was decomposed by wet ashing and then diluted to 200.0 mL in a volumetric flask. The analysis was completed by treating aliquots of this solution as indicated.
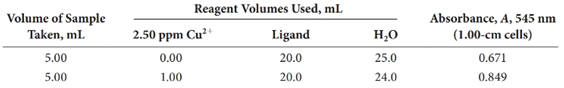
Calculate the percentage of copper in the sample.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
For the gravimetric determination of manganese, 1.8762 g of sample containing Mn was dissolved in acid solution and made up to 100 mL in a calibrated volumetric flask. A 25.00 mL aliquot of this solution was transferred to a beaker. The solution was heated to boiling and chemical substances required were added till precipitating MnNH4PO4. After filtering, rinsing, and igniting, 0.5365 g of Mn2P2O7 was obtained. Calculate the % manganese in the sample (Mn: 55, P: 31, O: 16 g/mol).
Chapter 14 Solutions
Principles of Instrumental Analysis
Ch. 14 - Prob. 14.1QAPCh. 14 - A 0.4740-g pesticide sample was decomposed by wet...Ch. 14 - Sketch a photometric titration curve for the...Ch. 14 - Prob. 14.4QAPCh. 14 - Prob. 14.5QAPCh. 14 - The accompanying data (1.00-cm cells) were...Ch. 14 - A 3.03-g petroleum specimen was decomposed by wet...Ch. 14 - Prob. 14.8QAPCh. 14 - Prob. 14.9QAPCh. 14 - The acid-base indicator HIn undergoes the...
Ch. 14 - Prob. 14.11QAPCh. 14 - Prob. 14.12QAPCh. 14 - Copper(II) forms a 1:1 complex with the organic...Ch. 14 - Aluminum forms a 1:1 complex with...Ch. 14 - Prob. 14.15QAPCh. 14 - Prob. 14.16QAPCh. 14 - Prob. 14.17QAPCh. 14 - Prob. 14.18QAPCh. 14 - Prob. 14.19QAPCh. 14 - Given the Information that...Ch. 14 - Prob. 14.21QAPCh. 14 - Mixing the chelating reagent B with Ni(II) forms...Ch. 14 - Prob. 14.23QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write balanced chemical equations for the following reactions: (a) metallic aluminum burned in air (b) elemental aluminum heated in an atmosphere of Chlorine (c) aluminum heated in hydrogen bromide gas (d) aluminum hydroxide added to a solution of nitric acidarrow_forwardA 2.5624-g sample of a pure solid alkali metal chloride is dissolved in water and treated with excess silver nitrate. The resulting precipitate, filtered and dried, weighs 3.03707 g. What was the percent by mass of chloride ion in the original compound? What is the identity of the salt?arrow_forwardA chemist dissolves a 1.497-g sample of a type of metal (an alloy of Sn, Pb, Sb, and Cu) in nitric acid, and metastannic acid, H2SnO3, is precipitated. She heats the precipitate to drive off the water, which leaves 0.4909 g of tin(IV) oxide. What was the percentage of [in in the original sample?arrow_forward
- Complete and balance the following chemical equations: (a) hardening of plaster containing slaked lime. Ca(OH)2+CO2 (b) removal of sulfur dioxide from the ?ue gas of power plants. CaO+SO2 (c) the reaction of baking powder that produces carbon dioxide gas and causes bread to rise. NaHCO3+NaH2PO4arrow_forwardThe mineral rhodothrosite is manganese() carbonate. Write an overall, balanced equation for the reaction of the mineral with hydrochloric acid, and name the products.arrow_forward4.48 Elemental phosphorous is used in the semiconductor industry. It can be obtained from an ore called fluoroapatite via reaction with SiO2 and C: 4Ca5( PO4)3F+18SiO2+30C3P4+30CO+18CaSiO3+2CaF2 Suppose a particular semiconductor production plant requires 1500 kg of P4. If the recovery of P4 from this reaction is 73% efficient, what mass of fluoroapatite is needed?arrow_forward
- Phosphorus occurs naturally in the form of fluorapatite, CaF2 3Ca3(PO4)2. The dot indicates 1 part CaF2 to 3 parts Ca3(PO4)2. This mineral is reacted with an aqueous solution of H2SO4 in the preparation of a fertilizer. The products are phosphoric acid, hydrogen fluoride, and gypsum, CaSO4 2H2O. Write the balanced equation describing this process.arrow_forwardAssume that the radius of Earth is 6400 km, the crust is 50. km thick, the density of the crust is 3.5 g/cm3, and 25.7% of the crust is silicon by mass. Calculate the total mass of silicon in the crust of Earth.arrow_forwardComplete and balance the equations of the following reactions, each of which could be used to remove hydrogen sulfide from natural gas: (a) Ca(OH)2(s)+H2S(g) (b) Na2CO3(aq)+H2S(g)arrow_forward
- You are given a 1.50-g mixture of sodium nitrate and sodium chloride. You dissolve this mixture into 100 mL of water and then add an excess of 0.500 M silver nitrate solution. You produce a white solid, which you then collect, dry, and measure. The white solid has a mass of 0.641 g. a. If you had an extremely magnified view of the solution (to the atomic-molecular level), list the species you would see (include charges, if any). b. Write the balanced net ionic equation for the reaction that produces the solid. Include phases and charges. c. Calculate the percent sodium chloride in the original unknown mixture.arrow_forwardWhat is a half-reaction? Why must the number of electrons lost in the oxidation half-reaction equal the number of electrons gained in the reduction half-reaction? Summarize briefly the steps in the half-reaction method for balancing redox reactions. What two items must be balanced in a redox reaction (or any reaction)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
O-Level Chemistry | 16 | Qualitative Analysis [1/3]; Author: Bernard Ng;https://www.youtube.com/watch?v=oaU8dReeBgA;License: Standard YouTube License, CC-BY