24. Which of these statements is true when comparing compounds 10 and 11? HO 10 IL A) 11 is a stronger acid than 10 because when the phenolic OH proton is removed, the negative charge can delocalized out onto the nitro group through resonance. 26. What is the DU of compound 11? A) 5 B) 6 C) 7 o НО 11 B) 10 is a stronger acid than 11 because when the phenolic OH proton is removed, the negative charge can delocalized out onto the nitro group through resonance. C) 11 is a stronger acid than 10 because when the phenolic OH proton is removed, nitrogen is so negative it puts electron density into the ring. D) 4 D) 10 is a stronger acid than 11 because when when the phenolic OH proton is removed, nitrogen is so negative it puts electron density into the ring.
24. Which of these statements is true when comparing compounds 10 and 11? HO 10 IL A) 11 is a stronger acid than 10 because when the phenolic OH proton is removed, the negative charge can delocalized out onto the nitro group through resonance. 26. What is the DU of compound 11? A) 5 B) 6 C) 7 o НО 11 B) 10 is a stronger acid than 11 because when the phenolic OH proton is removed, the negative charge can delocalized out onto the nitro group through resonance. C) 11 is a stronger acid than 10 because when the phenolic OH proton is removed, nitrogen is so negative it puts electron density into the ring. D) 4 D) 10 is a stronger acid than 11 because when when the phenolic OH proton is removed, nitrogen is so negative it puts electron density into the ring.
Chapter17: Alcohols And Phenols
Section17.2: Properties Of Alcohols And Phenols
Problem 4P: Rank the following substances in order of increasing acidity: (a) (CH3)2CHOH, HC≡CH, (CF3)2CHOH,...
Related questions
Question
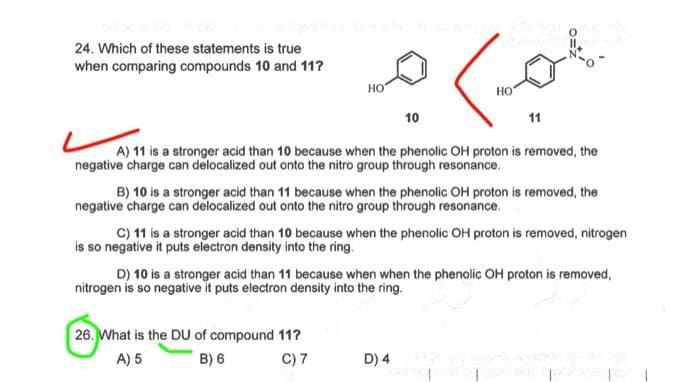
Transcribed Image Text:24. Which of these statements is true
when comparing compounds 10 and 11?
HO
10
ما
26. What is the DU of compound 11?
A) 5
B) 6
C) 7
НО
11
A) 11 is a stronger acid than 10 because when the phenolic OH proton is removed, the
negative charge can delocalized out onto the nitro group through resonance.
D) 4
IL
B) 10 is a stronger acid than 11 because when the phenolic OH proton is removed, the
negative charge can delocalized out onto the nitro group through resonance.
C) 11 is a stronger acid than 10 because when the phenolic OH proton is removed, nitrogen
is so negative it puts electron density into the ring.
D) 10 is a stronger acid than 11 because when when the phenolic OH proton is removed,
nitrogen is so negative it puts electron density into the ring.
199
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning