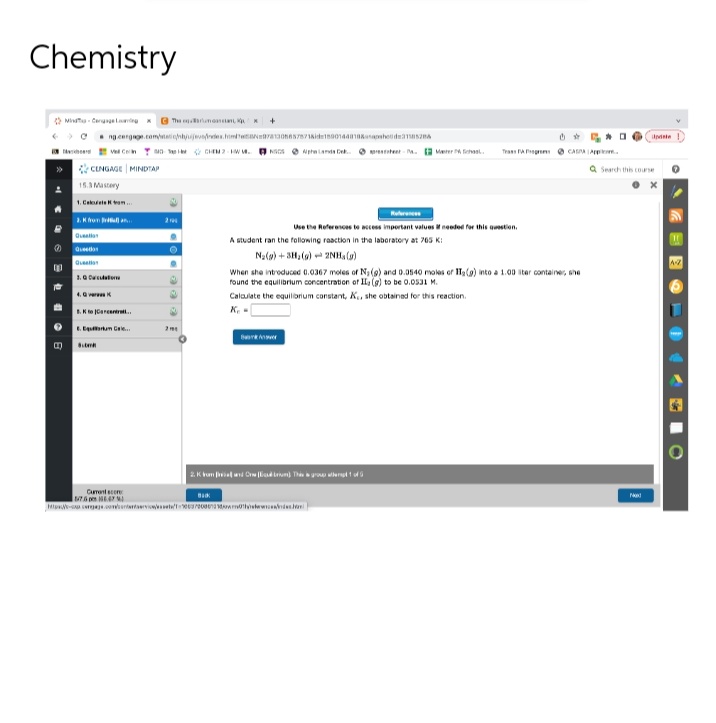
Chemistry B Mit-The G 1.0 ng cergage.com/taohjeves from 9781306657871Aide18001440108-ash12 CHIM-WM. CENGAGE MINDTAP 15.3 Mastery 1. Cam 1. K from rel come Bitm 8. Kto Carena.. 6. Em Gre... Currenc 76.47 2m + BACK NOSands Dk.M.M. Tras FA CASA.. a Search this course A student ran the following reaction in the laboratory at 765 K Na(s) + 3H₂()2NH, (g) When she introduced 0.0367 moles of N₁ (9) and 0.0540 moles or IT, (a) into a 1.00 ster container, she found the equilibrium concentration of II, (g) to be 0.0531 M. Calculate the equilibrium constant, K., she obtained for this reaction. K work ver H.com/00000 Use the References to access important values if needed for this question. 2. Kumand Thef Update P
Chemistry B Mit-The G 1.0 ng cergage.com/taohjeves from 9781306657871Aide18001440108-ash12 CHIM-WM. CENGAGE MINDTAP 15.3 Mastery 1. Cam 1. K from rel come Bitm 8. Kto Carena.. 6. Em Gre... Currenc 76.47 2m + BACK NOSands Dk.M.M. Tras FA CASA.. a Search this course A student ran the following reaction in the laboratory at 765 K Na(s) + 3H₂()2NH, (g) When she introduced 0.0367 moles of N₁ (9) and 0.0540 moles or IT, (a) into a 1.00 ster container, she found the equilibrium concentration of II, (g) to be 0.0531 M. Calculate the equilibrium constant, K., she obtained for this reaction. K work ver H.com/00000 Use the References to access important values if needed for this question. 2. Kumand Thef Update P
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Chemistry
Mid-Cen
for
» CENGAGE MINDTAP
15.3 Mastery
1. Calcule from...
3.K from Bra
>ng.cergage.com/tationbyujevades.homeN9781305657871&id=18001440188sphed152
Qu
3.0 Cacao
4. K
B.K to Cancentral...
E. Em Gaie...
Subm
The mean
Con CHIM-WM. NSS Lands Dek..beer-Merc
Cuent com
547647
200
O
+
201
Back
A student ran the following reaction in the laboratory at 765 K:
N₂(g) + 3H₂(g) + 2NH, (g)
Use the References to access important values if needed for this question.
Bankver
2. K kuma and CiThis group of
Tra
When she introduced 0.0367 moles of N₁ (9) and 0.0540 moles of IT₂(g) into a 1.00 liter container, she
found the equilibrium concentration of II, (g) to be 0.0531 M.
Calculate the equilibrium constant, K., she obtained for this reaction.
Ke
pc.com/content/t/1106/2006 0¹.
CASAA..
Update
Q Search this course
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

