25. Quartenary ammonium salts with hydrocarbon chains are used as detergent BECAUSE the presence of hydrophobic tail and ionic head facilitates its detergent action. 26. Hofmann elimination is a reaction between an unsubstituted amide and an alkaline solution of bromine BECAUSE the advantage of Hofmann rearrangement is that it yields pure primary amines 27. Nitriles undergo catalytic hydrogenation or reduction with LIAIH4 to yield primary amines BECAUSE this reaction is a technique for shortening carbon chain in an amine.
25. Quartenary ammonium salts with hydrocarbon chains are used as detergent BECAUSE the presence of hydrophobic tail and ionic head facilitates its detergent action. 26. Hofmann elimination is a reaction between an unsubstituted amide and an alkaline solution of bromine BECAUSE the advantage of Hofmann rearrangement is that it yields pure primary amines 27. Nitriles undergo catalytic hydrogenation or reduction with LIAIH4 to yield primary amines BECAUSE this reaction is a technique for shortening carbon chain in an amine.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter23: Addition To A Carbonyl
Section: Chapter Questions
Problem 25E
Related questions
Question
Give the reason why it is true or false

Transcribed Image Text:25. Quartenary ammonium salts with hydrocarbon chains are used as detergent
BECAUSE the presence of hydrophobic tail and ionic head facilitates its
detergent action.
26. Hofmann elimination is a reaction between an unsubstituted amide and an
alkaline solution of bromine BECAUSE the advantage of Hofmann
rearrangement is that it yields pure primary amines
27. Nitriles undergo catalytic hydrogenation or reduction with LIAIH4 to yield
primary amines BECAUSE this reaction is a technique for shortening carbon
chain in an amine.
Expert Solution
Step 1
25. The structure of quaternary ammonium salt is as shown below:
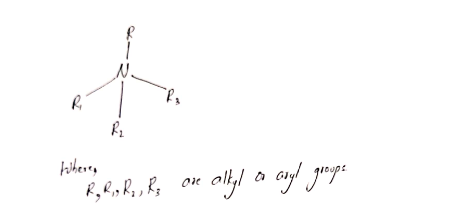
A detergent contains both hydrophobic and hydrophilic ends.
Hydrophobic part dissolves in the oil or grease and hydrophilic part dissolves in water.
Thus , they can remove the dirt and can act as detergents.
So, the given statement and reason both are true.
Step 2
26. Hofmann elimination is a reaction in which quaternary ammonium salts can be converted into alkenes by heating with Ag2O.
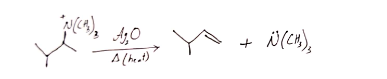
Hofmann rearrangement reaction involves the reaction between primary amides with Br2 in presence of NaOH and form primary amines.
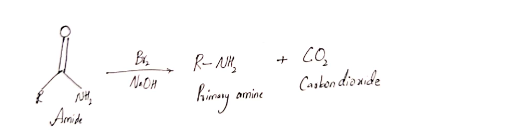
First statement is false, and second statement is true.
Step by step
Solved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
