2518-25 General, Settings Help Discussions List > View Topic Gas_Laws Cho 7 Unlocked: Monday, February 8, 2021 12:00 AM CST - Friday, February 12, 2021 11:30 PM CST. Must post first. * Subscribe 1. A gas has a volume of 159mL, a pressure os 1.25atm and a Temperature of 230C. If the pressure decreases to 0.95atm and the volume increases to 165mL, what is the new temperature of the gas in Celsius? 2. You have a container of N20 gas. It has a volume of 250.mL and pressure of 0.85atm at 36 OC. How many grams of N20 gas are present? 3. Determine the STRONGEST intermolecular forces (dipole-dipole, hydrogen bonding, or London Forces) for each of the following compounds: a) HF b) BF3 c) CH4 d) CH3OH e) NF3 You will also need to know how to use Boyle's, Charles' and Dalton's Gas Laws. I would suggest doing the practice problems and let me know if you have questions. Start a New Thread
2518-25 General, Settings Help Discussions List > View Topic Gas_Laws Cho 7 Unlocked: Monday, February 8, 2021 12:00 AM CST - Friday, February 12, 2021 11:30 PM CST. Must post first. * Subscribe 1. A gas has a volume of 159mL, a pressure os 1.25atm and a Temperature of 230C. If the pressure decreases to 0.95atm and the volume increases to 165mL, what is the new temperature of the gas in Celsius? 2. You have a container of N20 gas. It has a volume of 250.mL and pressure of 0.85atm at 36 OC. How many grams of N20 gas are present? 3. Determine the STRONGEST intermolecular forces (dipole-dipole, hydrogen bonding, or London Forces) for each of the following compounds: a) HF b) BF3 c) CH4 d) CH3OH e) NF3 You will also need to know how to use Boyle's, Charles' and Dalton's Gas Laws. I would suggest doing the practice problems and let me know if you have questions. Start a New Thread
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
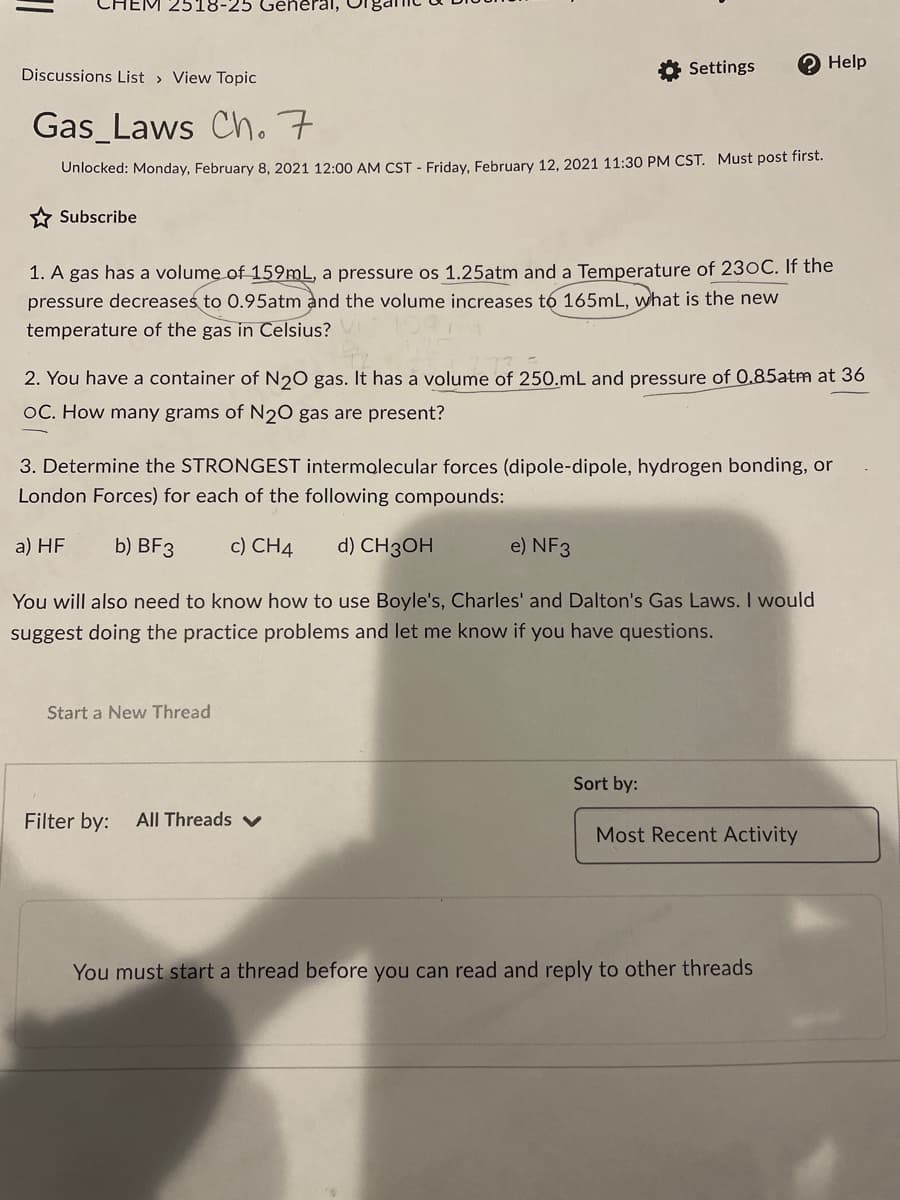
Transcribed Image Text:2518-25 General,
Settings
О Help
Discussions List > View Topic
Gas_Laws Cho 7
Unlocked: Monday, February 8, 2021 12:00 AM CST - Friday, February 12, 2021 11:30 PM CST. Must post first.
* Subscribe
1. A gas has a volume of 159mL, a pressure os 1.25atm and a Temperature of 230C. If the
pressure decreases to 0.95atm and the volume increases tó 165mL, what is the new
temperature of the gas in Celsius?
2. You have a container of N20 gas. It has a volume of 250.mL and pressure of 0.85atm at 36
OC. How many grams of N2O gas are present?
3. Determine the STRONGEST intermolecular forces (dipole-dipole, hydrogen bonding, or
London Forces) for each of the following compounds:
a) HF
b) BF3
c) CH4
d) CH3OH
e) NF3
You will also need to know how to use Boyle's, Charles' and Dalton's Gas Laws. I would
suggest doing the practice problems and let me know if you have questions.
Start a New Thread
Sort by:
Filter by:
All Threads v
Most Recent Activity
You must start a thread before you can read and reply to other threads
Expert Solution
Step 1
In the given question we have to calculate temperature after change in pressure and volume of gas,
by using ideal gas equation, PV=nRT

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning