28) How many moles contain 6.022 × 1023 lithium atoms? A) 2 moles B) 1 mole C) 6.022 x 1023 moles 29) How many moles of iron(II) sulfate are contained in 4 .657 g FeSO, ? B) 0.03066 C) 0.4657 A) 1.245 D) 151.9 30) How many moles contain 1.022 x 1023 copper atoms? A) 0.1697 mol B) 1.697 mol C) 0.01697 mol
28) How many moles contain 6.022 × 1023 lithium atoms? A) 2 moles B) 1 mole C) 6.022 x 1023 moles 29) How many moles of iron(II) sulfate are contained in 4 .657 g FeSO, ? B) 0.03066 C) 0.4657 A) 1.245 D) 151.9 30) How many moles contain 1.022 x 1023 copper atoms? A) 0.1697 mol B) 1.697 mol C) 0.01697 mol
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter3: Molecules, Moles, And Chemical Equations
Section: Chapter Questions
Problem 3.79PAE: 3.79 Consider two samples. Sample A contains 2 moles of N2 and 1 mole of O2, and Sample B contains 1...
Related questions
Question
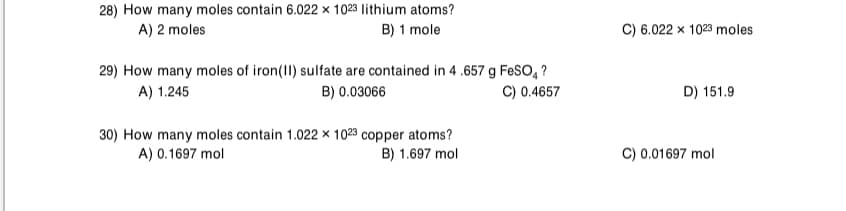
Transcribed Image Text:28) How many moles contain 6.022 × 1023 lithium atoms?
A) 2 moles
B) 1 mole
C) 6.022 x 1023 moles
29) How many moles of iron(II) sulfate are contained in 4 .657 g FeSO, ?
B) 0.03066
A) 1.245
C) 0.4657
D) 151.9
30) How many moles contain 1.022 × 1023 copper atoms?
A) 0.1697 mol
C) 0.01697 mol
B) 1.697 mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
