3: In the second part of the experiment, a solution containing 1.60x10-³ M Fe³+ and 4.00x10-4 M SCN was created, and the absorbance was recorded as 0.242 once the solution reached equilibrium. on, calculate the equilibrium concentration of FeNCS²+ present. 4: Using an ICE table, determine the equilibrium concentrations of Fe³+ and SCN in the previous question. Then, use the equilibrium concentrations of Fe³+, SCN", and FeNCS²+ to calculate Keq for the chemical reaction.
3: In the second part of the experiment, a solution containing 1.60x10-³ M Fe³+ and 4.00x10-4 M SCN was created, and the absorbance was recorded as 0.242 once the solution reached equilibrium. on, calculate the equilibrium concentration of FeNCS²+ present. 4: Using an ICE table, determine the equilibrium concentrations of Fe³+ and SCN in the previous question. Then, use the equilibrium concentrations of Fe³+, SCN", and FeNCS²+ to calculate Keq for the chemical reaction.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter20: Molecular Spectroscopy And Photochemistry
Section: Chapter Questions
Problem 67CP
Related questions
Question
See image below
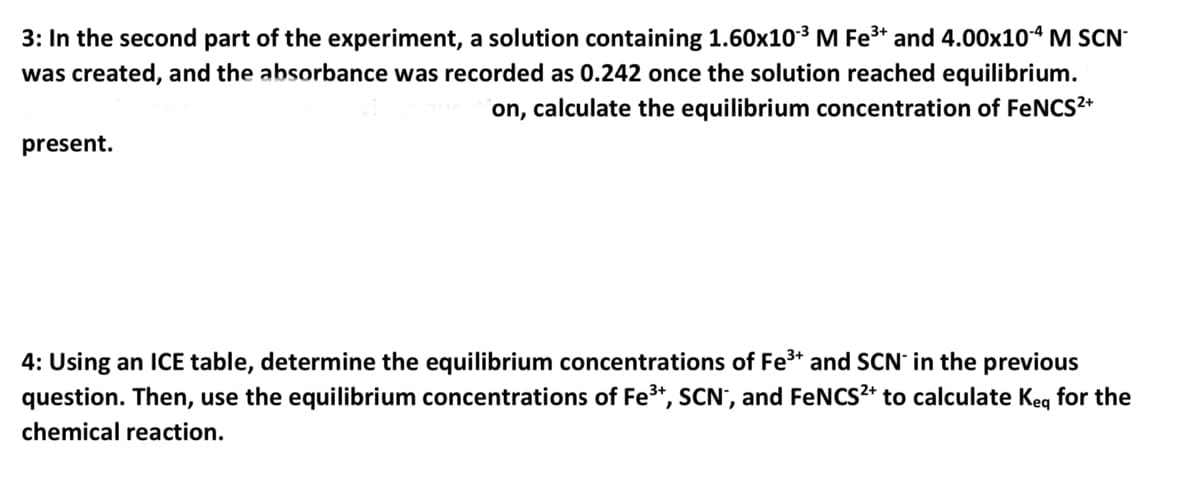
Transcribed Image Text:3: In the second part of the experiment, a solution containing 1.60x10³ M Fe³+ and 4.00x10-4 M SCN
was created, and the absorbance was recorded as 0.242 once the solution reached equilibrium.
on, calculate the equilibrium concentration of FeNCS²+
present.
4: Using an ICE table, determine the equilibrium concentrations of Fe³+ and SCN in the previous
question. Then, use the equilibrium concentrations of Fe³+, SCN*, and FeNCS²+ to calculate Keq for the
chemical reaction.
![Sample
Blank
S2
S3
$4
$5
S6
S7
S8
S9
$10
Stock of Fe
Stock of SC
Volume Fe3+, mL
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000
[Fe3+] M
0.200 M
2.00E-03 M
0.200
0.198
0.195
0.193
0.190
0.188
0.186
0.184
0.182
0.180
Volume SCN-, mL [SCN-] M
0.0000
0.0250
0.0500
0.0750
0.1000
0.1250
0.1500
0.1750
0.2000
0.2250
0.00E+00
2.47E-05
4.88E-05
7.23E-05
9.52E-05
1.18E-04
1.40E-04
1.61E-04
1.82E-04
2.02E-04
Absorbamce
0
0.097
0.214
0.313
0.408
0.509
0.599
0.688
0.771
0.858](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fdee8ae33-4eca-4bcd-9517-a19806391164%2F2155a6a9-4497-4f38-9cff-58a149665bbe%2Foajpt2_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Sample
Blank
S2
S3
$4
$5
S6
S7
S8
S9
$10
Stock of Fe
Stock of SC
Volume Fe3+, mL
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000
2.000
[Fe3+] M
0.200 M
2.00E-03 M
0.200
0.198
0.195
0.193
0.190
0.188
0.186
0.184
0.182
0.180
Volume SCN-, mL [SCN-] M
0.0000
0.0250
0.0500
0.0750
0.1000
0.1250
0.1500
0.1750
0.2000
0.2250
0.00E+00
2.47E-05
4.88E-05
7.23E-05
9.52E-05
1.18E-04
1.40E-04
1.61E-04
1.82E-04
2.02E-04
Absorbamce
0
0.097
0.214
0.313
0.408
0.509
0.599
0.688
0.771
0.858
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
