3 PHASE DIAGRAMS. Sketch the temperature-composition (“T-x") phase diagram for acetone, (CH3)2 CO), and chlo- roform, CHCl3, mixtures based on the following data- ⚫ Boiling point(Bp) of pure acetone: 56.2°C • • • Bp of pure chloroform: 61.2°C Bp of the acetone-chloroform azeotrope: 64.7°C Composition of the azeotrope, by weight: 21% acetone, 79% chloroform Atomic weights (in g mol¯¹): H: 1.010, C: 12.01, O: 16.00, Cl: 35.45 Plot the mole fraction of acetone, CHCl 3 on the x-axis On your sketch of the phase diagram determine the composition of the vapour phase and the liquid phase for a system at 61.2°C having an overall acetone mole fraction of ZA = 0.80.
3 PHASE DIAGRAMS. Sketch the temperature-composition (“T-x") phase diagram for acetone, (CH3)2 CO), and chlo- roform, CHCl3, mixtures based on the following data- ⚫ Boiling point(Bp) of pure acetone: 56.2°C • • • Bp of pure chloroform: 61.2°C Bp of the acetone-chloroform azeotrope: 64.7°C Composition of the azeotrope, by weight: 21% acetone, 79% chloroform Atomic weights (in g mol¯¹): H: 1.010, C: 12.01, O: 16.00, Cl: 35.45 Plot the mole fraction of acetone, CHCl 3 on the x-axis On your sketch of the phase diagram determine the composition of the vapour phase and the liquid phase for a system at 61.2°C having an overall acetone mole fraction of ZA = 0.80.
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter5: Distillation
Section: Chapter Questions
Problem 4Q
Related questions
Question
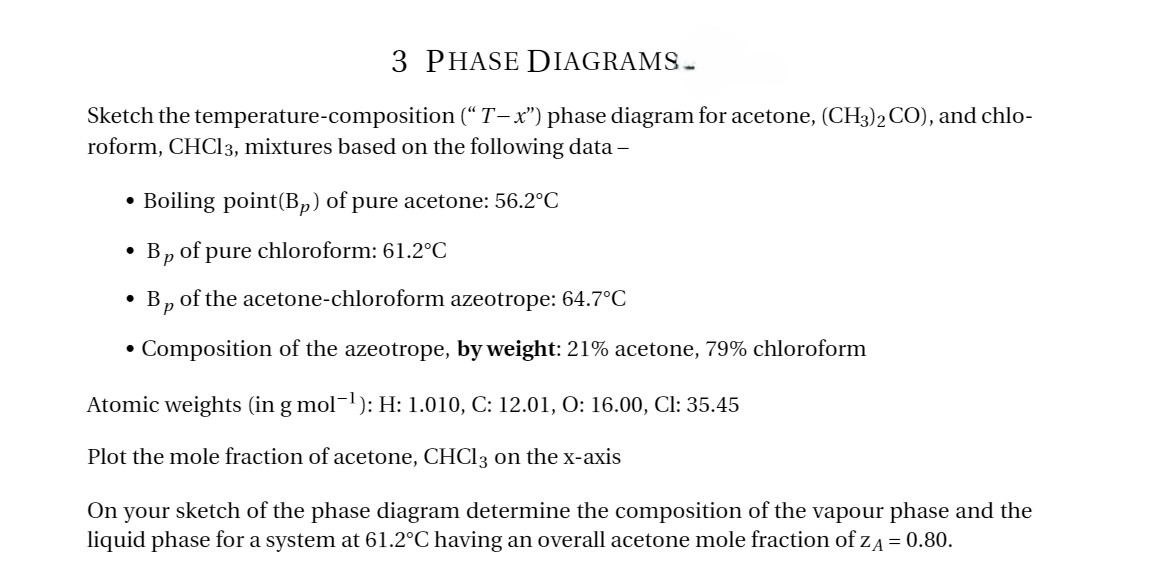
Transcribed Image Text:3 PHASE DIAGRAMS.
Sketch the temperature-composition (“T-x") phase diagram for acetone, (CH3)2 CO), and chlo-
roform, CHCl3, mixtures based on the following data-
⚫ Boiling point(Bp) of pure acetone: 56.2°C
•
•
•
Bp of pure chloroform: 61.2°C
Bp of the acetone-chloroform azeotrope: 64.7°C
Composition of the azeotrope, by weight: 21% acetone, 79% chloroform
Atomic weights (in g mol¯¹): H: 1.010, C: 12.01, O: 16.00, Cl: 35.45
Plot the mole fraction of acetone, CHCl 3 on the x-axis
On your sketch of the phase diagram determine the composition of the vapour phase and the
liquid phase for a system at 61.2°C having an overall acetone mole fraction of ZA = 0.80.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole