General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter11: States Of Matter; Liquids And Solids
Section: Chapter Questions
Problem 11.157QP: Nanotechnology, or technology utilizing 1100 nm sized particles, has rapidly expanded in the past...
Related questions
Question
100%
hello please help
Transcribed Image Text:le
Edit
View
History
Bookmarks
Profiles
Tab
Window
Help
L-C
D [Anir
A (1) t 3 sScho
Hom
Disn
- John
h Hulu
Oum
E Poet
Poet
O Spot
- SGM
= OX
Moc
Oum
docs.google.com/document/d/177iqtl-tjdHAJE3TcRdqoheH7eGuKBLLFybmbFPqaPU/edit
ny Diallo - Hon. Chem. SGM Balancing Equations
it View Insert Format Tools Add-ons Help
Last edit was 2 days ago
TUR
ア
100%
Normal text
Arial
15
+
BIU A
= E - E , E E
3
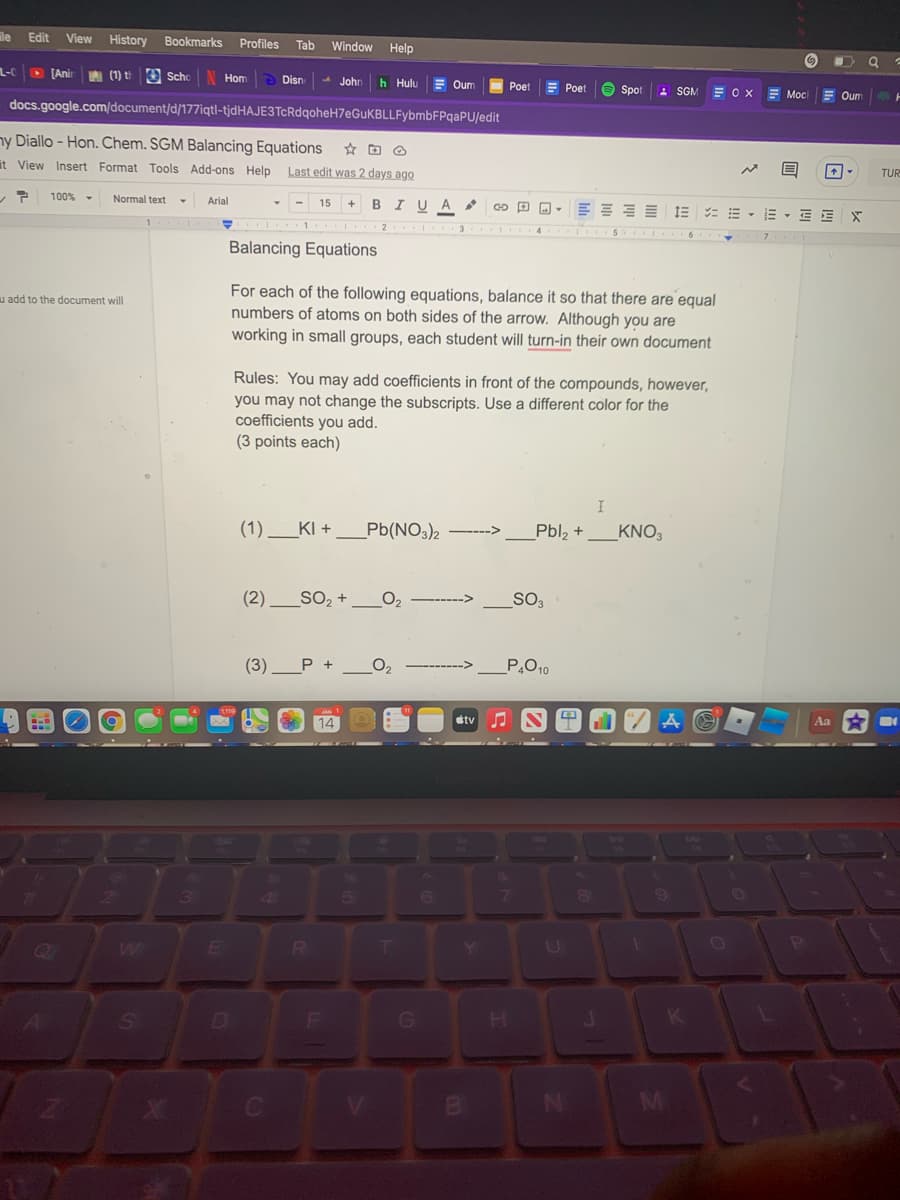
Balancing Equations
For each of the following equations, balance it so that there are equal
numbers of atoms on both sides of the arrow. Although you are
working in small groups, each student will turn-in their own document
u add to the document will
Rules: You may add coefficients in front of the compounds, however,
you may not change the subscripts. Use a different color for the
coefficients you add.
(3 points each)
I
(1).
KI +
Pb(NO3)2
Pbl, +
_KNO3
---->
(2) _SO, +
O2
SO3
(3)
P +
P.O10
14
étv
Aa
13
W
Y
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,